| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346109 | Tetrahedron: Asymmetry | 2005 | 11 Pages |
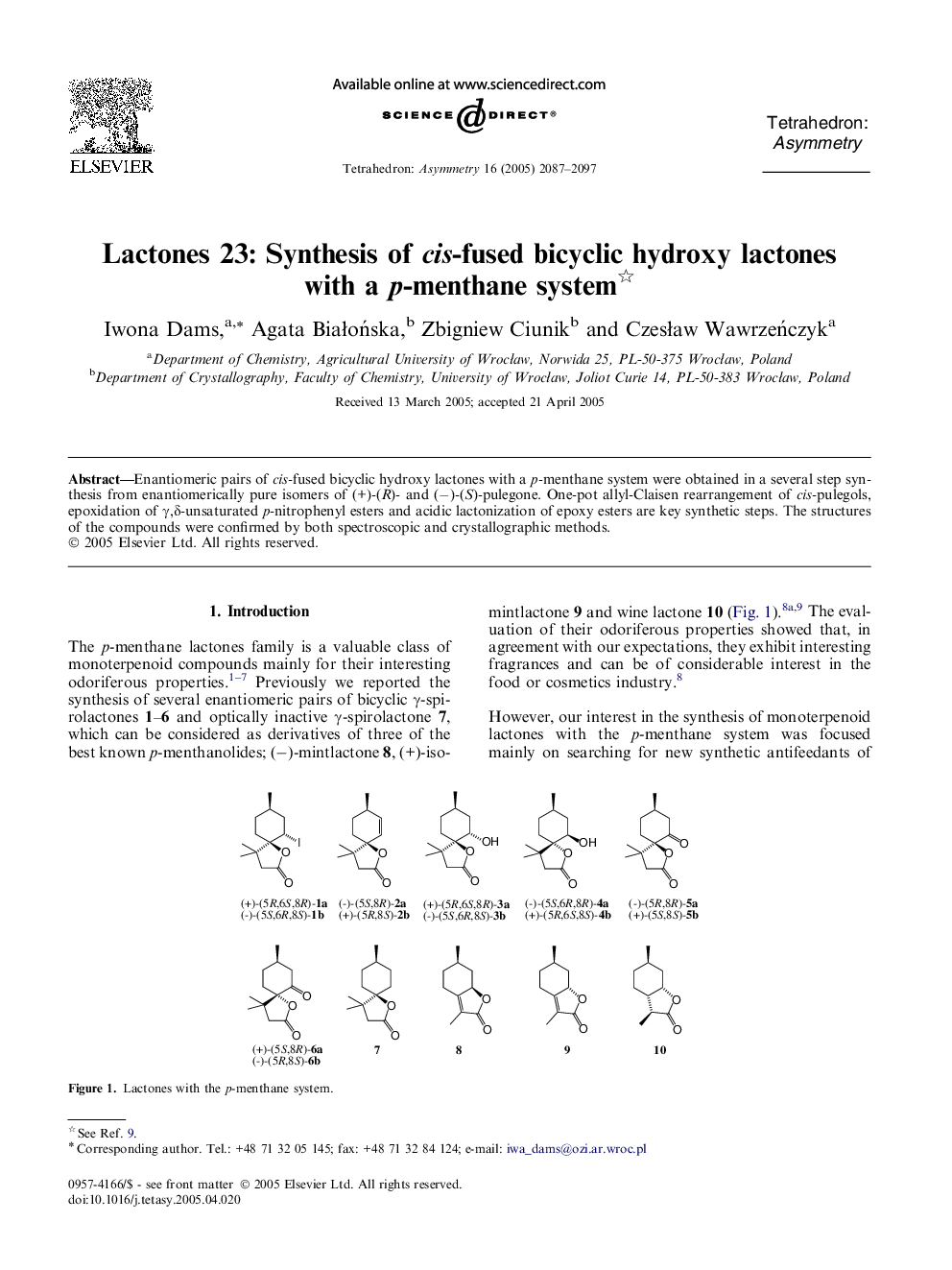
Enantiomeric pairs of cis-fused bicyclic hydroxy lactones with a p-menthane system were obtained in a several step synthesis from enantiomerically pure isomers of (+)-(R)- and (−)-(S)-pulegone. One-pot allyl-Claisen rearrangement of cis-pulegols, epoxidation of γ,δ-unsaturated p-nitrophenyl esters and acidic lactonization of epoxy esters are key synthetic steps. The structures of the compounds were confirmed by both spectroscopic and crystallographic methods.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Ethyl (2′-isopropyliden-5′-methylcyclohex-1′-yl)acetateC14H24O2Ee = 100%[α]D25=+30.5 (c 3.16, acetone)Absolute configuration:(1′R,5′R)
p-Nitrophenyl (2′-isoporopyliden-5′-methylcyclohex-1′-yl)acetateC18H23NO4Ee = 100%[α]D25=+14.3 (c 3.45, acetone)Absolute configuration:(1′R,5′R)
p-Nitrophenyl (2′-isopropyliden-5′-methylcyclohex-1′-yl)acetateC18H23NO4Ee = 100%[α]D25=-73.9 (c 1.29, acetone)Absolute configuration:(1′S,5′R)
p-Nitrophenyl (2′,7′-epoxy-2′-isoporopyl-5′-methylcyclohex-1′-yl)acetateC18H23NO4Ee = 100%[α]D25=+37.5 (c 3.11, acetone)Absolute configuration:(1′R,2′R,5′R)
1-Hydroxy-2,2,8-trimethyl-3-oxabicyclo[4.4.0]decan-4-oneC12H20O3Ee = 100%[α]D25=+43.8 (c 1.46, acetone)Absolute configuration:(1S,6R,8R)
1-(1′-Hydroxy-1′-methylethyl)-4-methyl-9-oxabicyclo[4.3.0]nonan-8-oneC12H20O3Ee = 100%[α]D25=-21.0 (c 1.52, acetone)Absolute configuration:(1R,4R,6R)
p-Nitrophenyl (2′,7′-epoxy-2′-isoporopyl-5′-methylcyclohex-1′- yl)acetateC18H23NO4Ee = 100%[α]D27=-44.1 (c 1.42, acetone)Absolute configuration: (1′S,2′S,5′R)
p-Nitrophenyl (2′,7′-epoxy-2′-isoporopyl-5′-methylcyclohex-1′-yl)acetateC18H23NO4Ee = 100%[α]D27=-14.3 (c 0.97, acetone)Absolute configuration: (1′S,2′R,5′R)