| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346135 | Tetrahedron: Asymmetry | 2014 | 8 Pages |
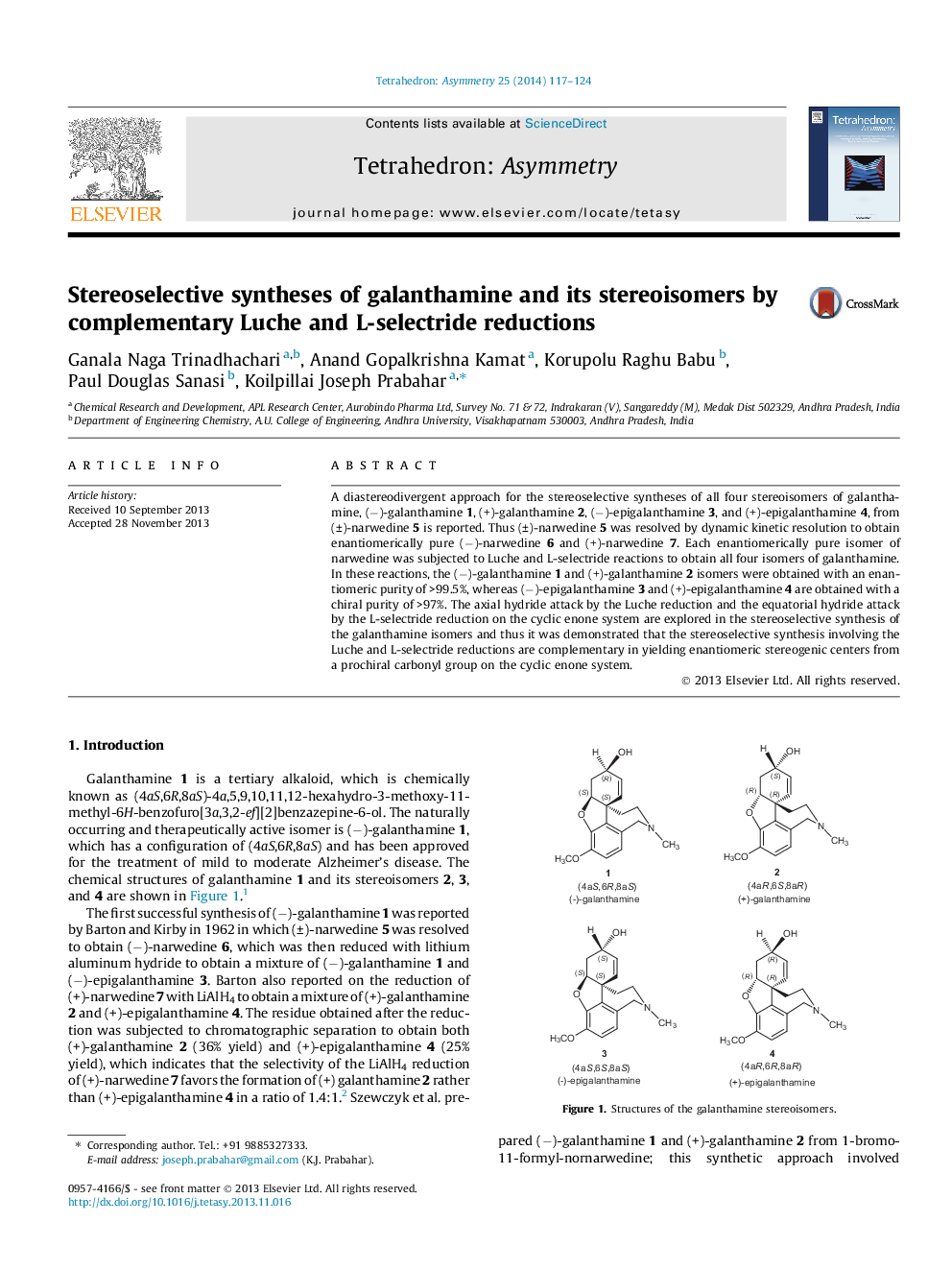
A diastereodivergent approach for the stereoselective syntheses of all four stereoisomers of galanthamine, (−)-galanthamine 1, (+)-galanthamine 2, (−)-epigalanthamine 3, and (+)-epigalanthamine 4, from (±)-narwedine 5 is reported. Thus (±)-narwedine 5 was resolved by dynamic kinetic resolution to obtain enantiomerically pure (−)-narwedine 6 and (+)-narwedine 7. Each enantiomerically pure isomer of narwedine was subjected to Luche and L-selectride reactions to obtain all four isomers of galanthamine. In these reactions, the (−)-galanthamine 1 and (+)-galanthamine 2 isomers were obtained with an enantiomeric purity of >99.5%, whereas (−)-epigalanthamine 3 and (+)-epigalanthamine 4 are obtained with a chiral purity of >97%. The axial hydride attack by the Luche reduction and the equatorial hydride attack by the L-selectride reduction on the cyclic enone system are explored in the stereoselective synthesis of the galanthamine isomers and thus it was demonstrated that the stereoselective synthesis involving the Luche and L-selectride reductions are complementary in yielding enantiomeric stereogenic centers from a prochiral carbonyl group on the cyclic enone system.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(4aS,8aS)-4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2]benzazepin-6-oneC17H19NO3[α]D25=-409.1 (c 1, CHCl3)Source of chirality: Kinetic dynamic resolution of (±)-narwedineAbsolute configuration: (4aS,8aS)
(4aR,8aR)-4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2]benzazepin-6-oneC17H19NO3[α]D25=+412.3 (c 1, CHCl3)Source of chirality: Kinetic dynamic resolution of (±)-narwedineAbsolute configuration: (4aR,8aR)
(4aS,6R,8aS)-4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2] benzazepine-6-ol hydrobromideC17H21NO3·HBr[α]D25=-96.5 (c 1, H2O)Source of chirality: Asymmetric reductionAbsolute configuration: [4aS,6R,8aS]
(4aR,6S,8aR)-4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2] benzazepine-6-ol hydrobromideC17H21NO3·HBr[α]D25=+97.7 (c 1, H2O)Source of chirality: Asymmetric reductionAbsolute configuration: (4aR,6S,8aR)
(4aS,6S,8aS)-4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2] benzazepine-6-olC17H21NO3[α]D25=-229.9 (c 1, CHCl3)Source of chirality: Asymmetric reductionAbsolute configuration: (4aS,6S,8aS)
(4aR,6R,8aR)-4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2] benzazepine-6-olC17H21NO3[α]D25=-232.6 (c 1, CHCl3)Source of chirality: Asymmetric reductionAbsolute configuration: [4aR,6R,8aR]