| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346172 | Tetrahedron: Asymmetry | 2012 | 5 Pages |
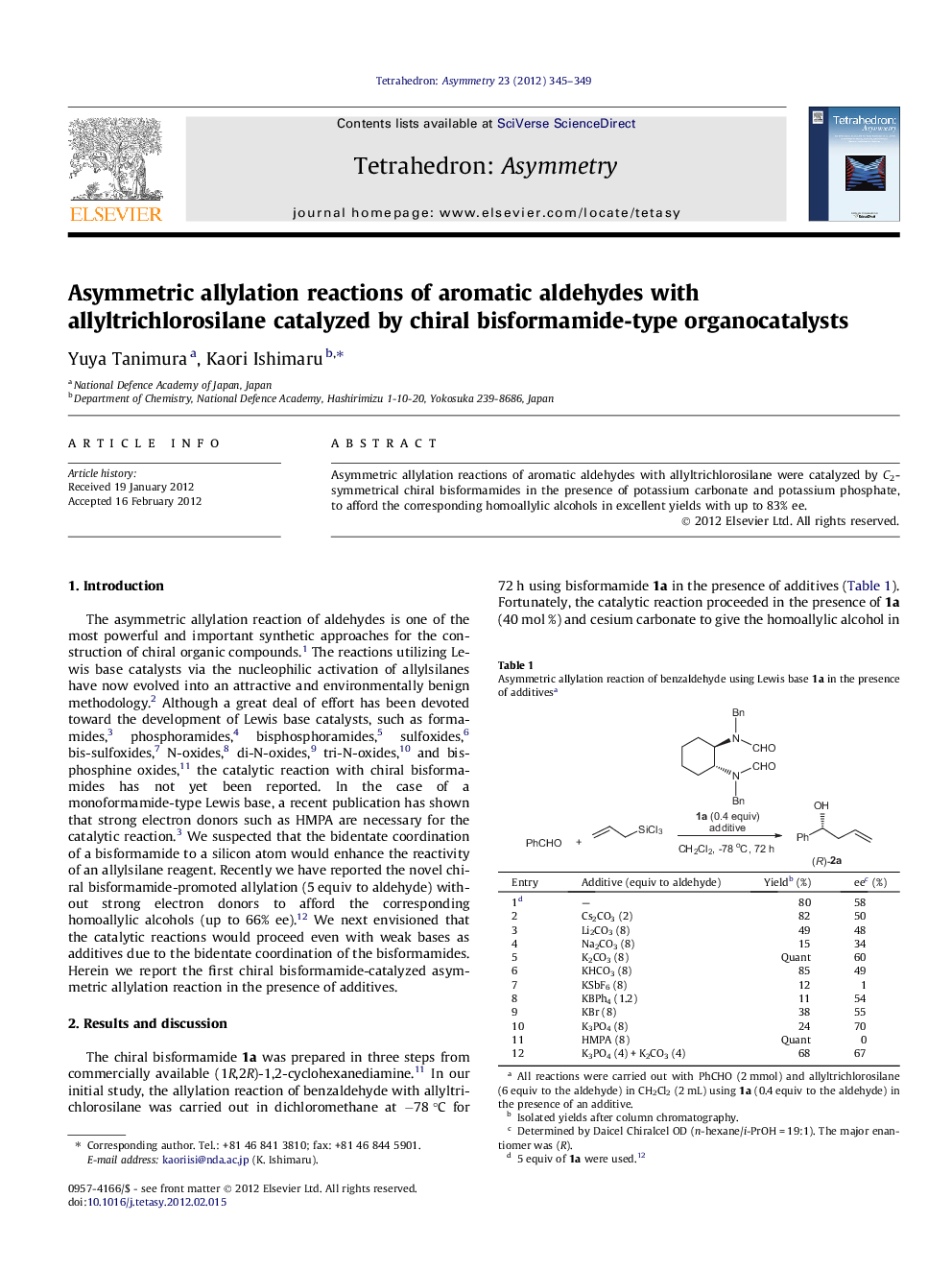
Asymmetric allylation reactions of aromatic aldehydes with allyltrichlorosilane were catalyzed by C2-symmetrical chiral bisformamides in the presence of potassium carbonate and potassium phosphate, to afford the corresponding homoallylic alcohols in excellent yields with up to 83% ee.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(1R,2R)-N,N′-Bisformyl-N,N′-(3-methylbenzyl)-1,2-cyclohexanediamineC24H30N2O2[α]D15=+16.0 (c 1.0, CHCl3)Source of chirality: (R,R)-1,2-cyclohexanediamineAbsolute configuration: (1R,2R)
(1R,2R)-N,N′-Bisformyl-N,N′-bis(2,3-dimethylbenzyl)-1,2-cyclohexanediamineC26H34N2O2[α]D15=-63.9 (c 1.0, CHCl3)Source of chirality: (R,R)-1,2-cyclohexanediamineAbsolute configuration: (1R,2R)
(1R,2R)-N,N′-Bisformyl-N,N′-bis(2,4,5-trimethylbenzyl)-1,2-cyclohexanediamineC28H38N2O2[α]D15=-31.8 (c 1.0, CHCl3)Source of chirality: (R,R)-1,2-cyclohexanediamineAbsolute configuration: (1R,2R)
(1R,2R)-N,N′-Bisformyl-N,N′-bis(2-isopropylbenzyl)-1,2-cyclohexanediamineC28H38N2O2[α]D15=-63.0 (c 1.0, CHCl3)Source of chirality: (R,R)-1,2-cyclohexanediamineAbsolute configuration: (1R,2R)
(1R,2R)-N,N′-Bisformyl-N,N′-bis(2-sec-butylbenzyl)-1,2-cyclohexanediamineC30H42N2O2[α]D15=+57.0 (c 1.0, CHCl3)Source of chirality: (R,R)-1,2-cyclohexanediamineAbsolute configuration: (1R,2R)
(1R,2R)-N,N′-Bisformyl-N,N′-bis(2-tert-butylbenzyl)-1,2-cyclohexanediamineC30H42N2O2[α]D15=-19.8 (c 1.0, CHCl3)Source of chirality: (R,R)-1,2-cyclohexanediamineAbsolute configuration: (1R,2R)
(1R,2R)-N,N′-Bisformyl-N,N′-bis[2-(3,5-dimethylphenyl)benzyl]-1,2-cyclohexanediamineC38H42N2O2[α]D15=+22.9 (c 1.0, CHCl3)Source of chirality: (R,R)-1,2-cyclohexanediamineAbsolute configuration: (1R,2R)
(1R,2R)-N,N′-Bisformyl-N,N′-bis(1-naphthylmethyl)-1,2-cyclohexanediamineC30H30N2O2[α]D15=-11.7 (c 1.0, CHCl3)Source of chirality: (R,R)-1,2-cyclohexanediamineAbsolute configuration: (1R,2R)
(1R,2R)-N,N′-Bis(9-anthracenylmethyl)-N,N′-bisformyl-1,2-cyclohexanediamineC38H34N2O2[α]D15=-88.9 (c 1.0, CHCl3)Source of chirality: (R,R)-1,2-cyclohexanediamineAbsolute configuration: (1R,2R)
(1R,2R)-N,N′-Dibenzyl-N-formyl-N′-methyl-1,2-cyclohexanediamineC22H28N2O[α]D15=-15.2 (c 1.0, CHCl3)Source of chirality: (R,R)-1,2-cyclohexanediamineAbsolute configuration: (1R,2R)