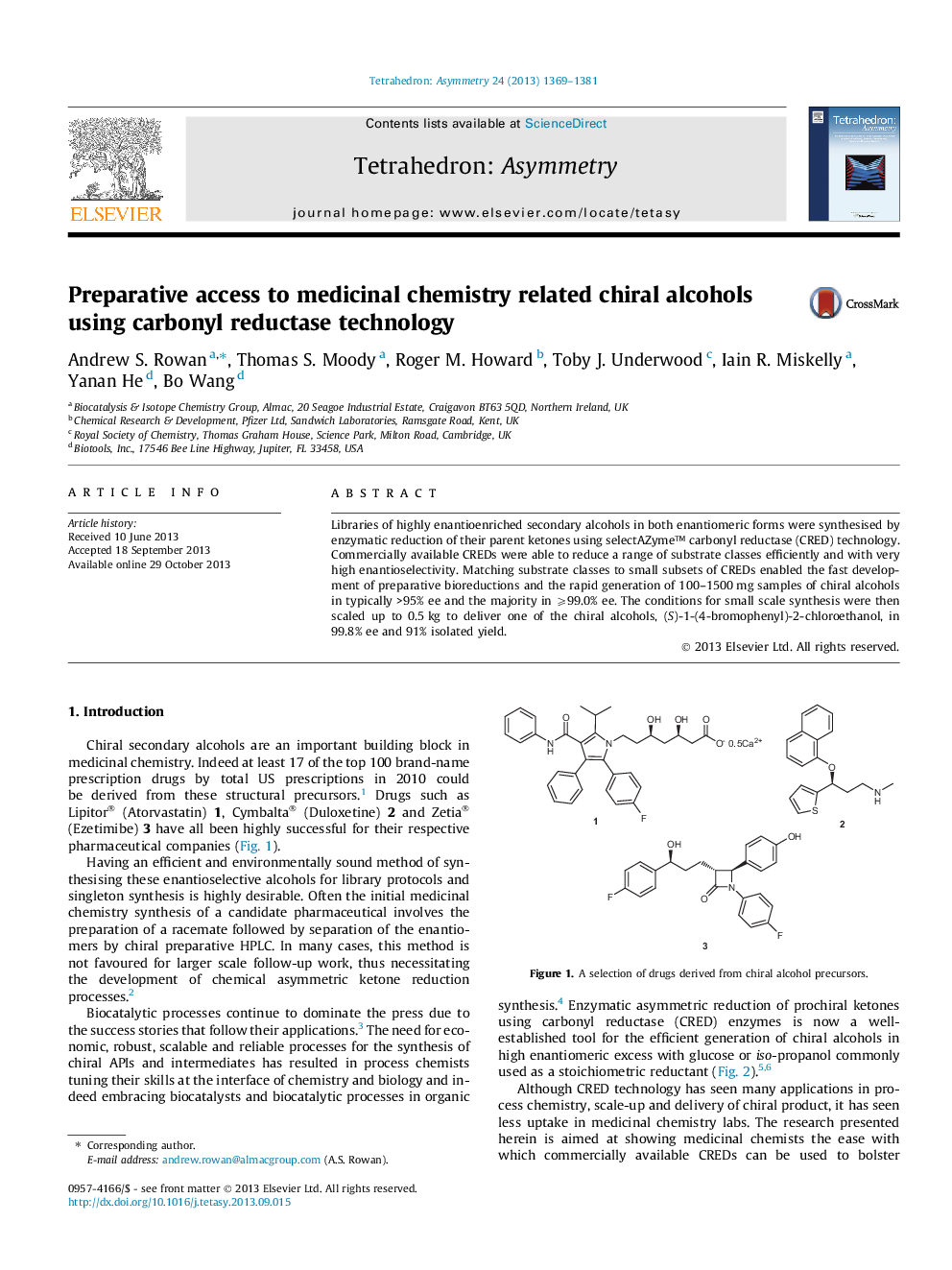
| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346284 | Tetrahedron: Asymmetry | 2013 | 13 Pages |
Libraries of highly enantioenriched secondary alcohols in both enantiomeric forms were synthesised by enzymatic reduction of their parent ketones using selectAZyme™ carbonyl reductase (CRED) technology. Commercially available CREDs were able to reduce a range of substrate classes efficiently and with very high enantioselectivity. Matching substrate classes to small subsets of CREDs enabled the fast development of preparative bioreductions and the rapid generation of 100–1500 mg samples of chiral alcohols in typically >95% ee and the majority in ⩾99.0% ee. The conditions for small scale synthesis were then scaled up to 0.5 kg to deliver one of the chiral alcohols, (S)-1-(4-bromophenyl)-2-chloroethanol, in 99.8% ee and 91% isolated yield.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-1-(3-Fluoro-4-methylphenyl)ethanolC9H9FOee = 98.3%[α]D20=-38.0 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(4-(1H-1,2,4-Triazol-1-yl)phenyl)ethanolC10H11N3Oee = 99.9%[α]D20 = −44.0 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(Pyrazin-2-yl)ethanolC6H8N2Oee = 99.8%[α]D20 = −29.4 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(3-Chloropyridin-4-yl)ethanolC7H8ClNOee = 99.9%[α]D20 = −66.5 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-3-(1-Hydroxyethyl)benzenesulfonamideC8H11NO3See = 99.8%[α]D20 = −29.5 (c 1.0, MeOH)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(3-Methylpyrazin-2-yl)ethanolC7H10N2Oee = 99.7%[α]D20 = −86.9 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(4-Methylthiazol-5-yl)ethanolC6H9NOSee = 99.8%[α]D20 = −35.3 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-2-Bromo-1-(4-(trifluoromethoxy)phenyl)ethanolC9H8BrF3O2ee = 96.9%[α]D20 = +33.2 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-2-Bromo-1-(3-fluoro-4-methoxyphenyl)ethanolC9H10BrFO2ee = 99.2%Unable to get optical rotation due to decomposition of productSource of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-3-(2-Bromo-1-hydroxyethyl)benzonitrileC9H8BrNOee = 97.6%[α]D20 = +43.5 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-2-Chloro-1-(2,4-difluorophenyl)ethanolC8H7ClF2Oee = 99.5%[α]D20 = +38.0 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(4-Bromophenyl)-2-chloroethanolC8H8BrClOee = 99.9%[α]D20 = +40.2 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(4-Bromophenyl)-2-chloroethanolC8H8BrClOee = 99.9%[α]D20 = +40.2 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-2-Chloro-1-(4-fluorophenyl)ethanolC8H8ClFOee = 98.4%[α]D20 = +52.8 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-2-Chloro-1-phenylethanolC8H9ClOee = 96.6%[α]D20 = +57.8 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-5,6,7,8-Tetrahydroisoquinolin-5-olC9H11NOee = 98.3%[α]D20 = +51.7 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-Chroman-4-olC9H10O2ee = 93.5%[α]D20 = −65.1 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-5-Bromo-1,2,3,4-tetrahydronaphthalen-1-olC10H11BrOee = 90.9%[α]D20 = −4.0 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-6-Chloro-1-(methylsulfonyl)-1,2,3,4-tetrahydroquinolin-4-olC10H12ClNO3See = 99.3%[α]D20 = −5.7 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-Isochroman-4-olC9H10O2ee = 96.0%[α]D20 = +17.6 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-2-Chloro-6,7-dihydro-5H-cyclopenta[b]pyridin-7-olC8H8ClNOEe = 99.5%[α]D20=+2.4 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-3-Hydroxy-2,3-dihydrobenzo[b]thiophene 1,1-dioxideC8H8O3See = 98.4%[α]D20 = −55.4 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-3-(4-Bromophenyl)-3-hydroxypropanenitrileC9H8BrNOee = 99.4%[α]D20 = −46.3 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-3-Hydroxy-3-phenylpropanenitrileC9H9NOEe = 100%[α]D20=-61.3 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-3-Hydroxy-3-(pyridin-4-yl)propanenitrileC8H8N2Oee = 99.6%[α]D20 = −10.6 (c 0.2, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(Pyrimidin-2-yl)piperidin-3-olC9H13N3OEe = 99.2%[α]D20=+44.4 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(Pyridin-2-yl)pyrrolidin-3-olC9H12N2Oee = 99.9%[α]D20 = +34.1 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(Pyrimidin-2-yl)pyrrolidin-3-olC8H11N3Oee = 99.9%[α]D20 = +68.5 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-(4-Bromophenyl)(cyclopropyl)methanolC10H11BrOee = 99.0%[α]D20 = +32.1 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-Cyclopropyl(pyridin-4-yl)methanolC9H11NOee = 99.9%[α]D20 = +32.7 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-2-Methyl-1-(pyridin-3-yl)propan-1-olC9H13NOee = 98.7%[α]D20 = −52.0 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(3-Chlorophenyl)-2-methylpropan-1-olC10H13ClOee = 97.7%[α]D20 = −31.6 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-2,2,2-Trifluoro-1-(6-fluoro-1-methyl-1H-indol-3-yl)ethanolC11H9F4NOee = 99.9%[α]D20 = +12.9 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(4-Chlorophenyl)-2,2,2-trifluoroethanolC8H6ClF3Oee = 98.7%[α]D20 = +26.1 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-1-(3-Chlorophenyl)-2,2,2-trifluoroethanolC8H6ClF3Oee = 99.6%[α]D20 = +23.1 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)
(S)-4-(2,2,2-Trifluoro-1-hydroxyethyl)benzoic acidC9H7F3O3ee = 93.4%[α]D20 = +24.5 (c 1.0, CHCl3)Source of chirality: asymmetric bioreductionAbsolute configuration: (S)