| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346286 | Tetrahedron: Asymmetry | 2013 | 6 Pages |
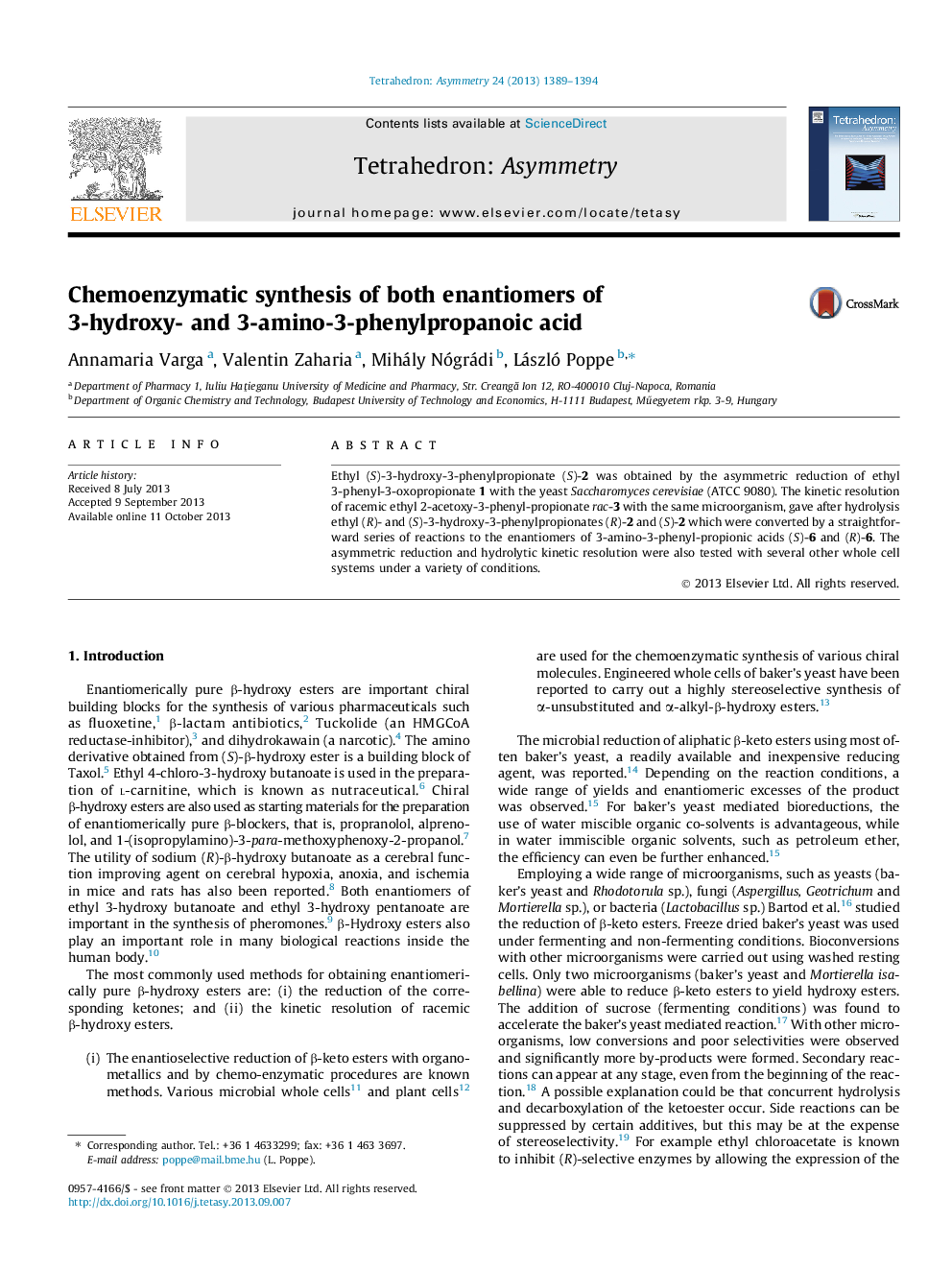
Ethyl (S)-3-hydroxy-3-phenylpropionate (S)-2 was obtained by the asymmetric reduction of ethyl 3-phenyl-3-oxopropionate 1 with the yeast Saccharomyces cerevisiae (ATCC 9080). The kinetic resolution of racemic ethyl 2-acetoxy-3-phenyl-propionate rac-3 with the same microorganism, gave after hydrolysis ethyl (R)- and (S)-3-hydroxy-3-phenylpropionates (R)-2 and (S)-2 which were converted by a straightforward series of reactions to the enantiomers of 3-amino-3-phenyl-propionic acids (S)-6 and (R)-6. The asymmetric reduction and hydrolytic kinetic resolution were also tested with several other whole cell systems under a variety of conditions.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Ethyl (S)-3-hydroxy-3-phenylpropanoateC11H14O3ee = 96% on Chiralpak IA/IB tandem HPLC columns[α]D25=-51.5 (c 1.5, CHCl3)Source of chirality: microbial reductionAbsolute configuration: (S)
Ethyl (S)-3-acetoxy-3-phenylpropanoateC13H16O4ee = 96% on β-PM GC column[α]D25=-1.1 (c 1.5, CHCl3)Source of chirality: microbial enantiomer selective hydrolysisAbsolute configuration: (S)
Ethyl (S)-3-amino-3-phenylpropanoateC11H15NO2ee = 96% on Chiracel OD-H HPLC column[α]D25=-21.8 (c 1.0, CHCl3)Source of chirality: microbial enantiomer selective hydrolysisAbsolute configuration: (S)
(S)-3-Amino-3-phenylpropanoic acidC9H11NO2ee = 96% on Chiracel IA HPLC column[α]D25=-7.7 (c 0.25, H2O)Source of chirality: microbial enantiomer selective hydrolysisAbsolute configuration: (S)