| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346406 | Tetrahedron: Asymmetry | 2011 | 6 Pages |
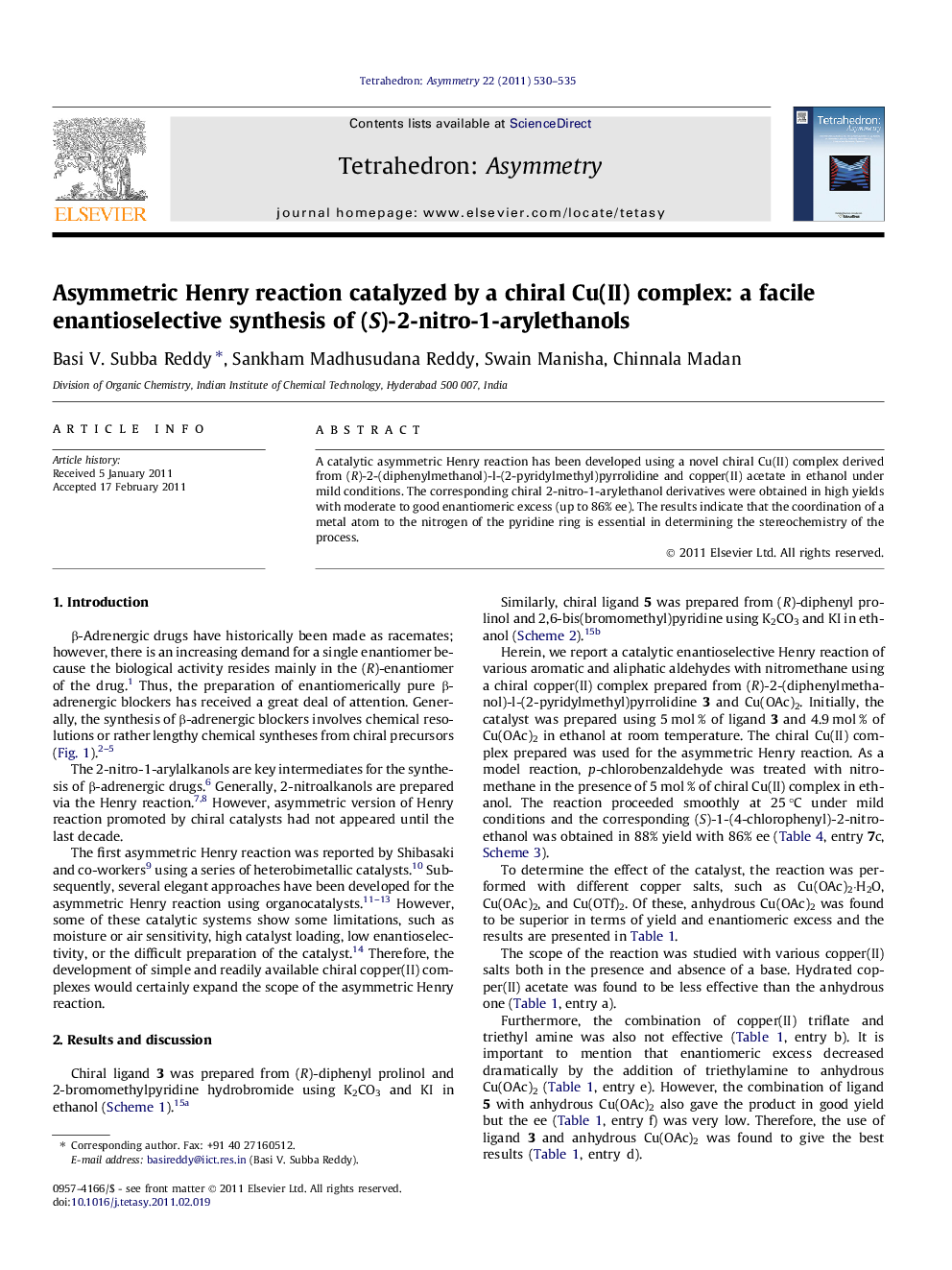
A catalytic asymmetric Henry reaction has been developed using a novel chiral Cu(II) complex derived from (R)-2-(diphenylmethanol)-l-(2-pyridylmethyl)pyrrolidine and copper(II) acetate in ethanol under mild conditions. The corresponding chiral 2-nitro-1-arylethanol derivatives were obtained in high yields with moderate to good enantiomeric excess (up to 86% ee). The results indicate that the coordination of a metal atom to the nitrogen of the pyridine ring is essential in determining the stereochemistry of the process.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-1-(4-Bromophenyl)-2-nitroethanolC8H8BrNO3Ee = 82% from HPLC[α]D=+63.2[α]D=+63.2 (c 0.60, CHCl3)Source of chirality: asymmetric synthesis
(S)-1-(2-Bromophenyl)-2-nitroethanolC8H8NO3Ee = 73% from HPLC[α]D=-27.3[α]D=-27.3 (c 1.12, CH2Cl2)Source of chirality: asymmetric synthesis
(S)-1-(4-Chlorophenyl)-2-nitroethanolC8H8ClNO3Ee = 86% from HPLC[α]D=+34.8[α]D=+34.8 (c 1.02, CH2Cl2)Source of chirality: asymmetric synthesis
(S)-1-(2-Chlorophenyl)-2-nitroethanolC8H8ClNO3Ee = 76% from HPLC[α]D=+44.2[α]D=+44.2 (c 0.46, CH2Cl2)Source of chirality: asymmetric synthesis
C8H7Cl2NO3(S)-1-(3,4-Dichlorophenyl)-2-nitroethanolEe = 74% from HPLC[α]D=+26.0[α]D=+26.0 (c 1.43, CH2Cl2)Source of chirality: asymmetric synthesis
C8H8FNO3(S)-1-(4-Fluorophenyl)-2-nitroethanolEe = 75% from HPLC[α]D=+28.0[α]D=+28.0 (c 0.62, CH2Cl2)Source of chirality: asymmetric synthesis
(S)-1-(2-Iodophenyl)-2-nitroethanolC8H8INO3Ee = 68% from HPLC[α]D=+23.2[α]D=+23.2 (c 1.02, CH2Cl2)Source of chirality: asymmetric synthesis
(S)-1-(4-Methoxyphenyl)-2-nitroethanolC9H11NO4Ee = 82% from HPLC[α]D=+33.8[α]D=+33.8 (c 1.02, CH2Cl2)Source of chirality: asymmetric synthesis
(S)-1-(3,4-Dimethoxyphenyl)-2-nitroethanolC10H13NO5Ee = 82% from HPLC[α]D=+28.1[α]D=+28.1 (c 1.2, CH2Cl2)Source of chirality: asymmetric synthesis
(S)-1-Phenyl-2-nitroethanolC8H9NO3Ee = 50% from HPLC[α]D=+19.3[α]D=+19.3 (c 0.48, CH2Cl2)Source of chirality: asymmetric synthesis
(S)-1-(4-Nitrophenyl)-2-nitroethanolC8H8N2O5Ee = 53% from HPLC[α]D=+18.6[α]D=+18.6 (c 0.68, CH2Cl2)Source of chirality: asymmetric synthesis
(S)-1-(2-Nitrophenyl)-2-nitroethanolC8H8N2O5Ee = 81% from HPLC[α]D=-189.8[α]D=-189.8 (c 0.58, CH2Cl2)Source of chirality: asymmetric synthesis
(S)-1-(1-Naphthyl)-2-nitroethanolC12H11NO3Ee = 81% from HPLC[α]D=+15.4[α]D=+15.4 (c 0.98, CH2Cl2)Source of chirality: asymmetric synthesis
(S)-1-Nitropentan-2-olC5H11NO3Ee = 77% from HPLC[α]D=+12.2[α]D=+12.2 (c 0.2, CHCl3)Source of chirality: asymmetric synthesis