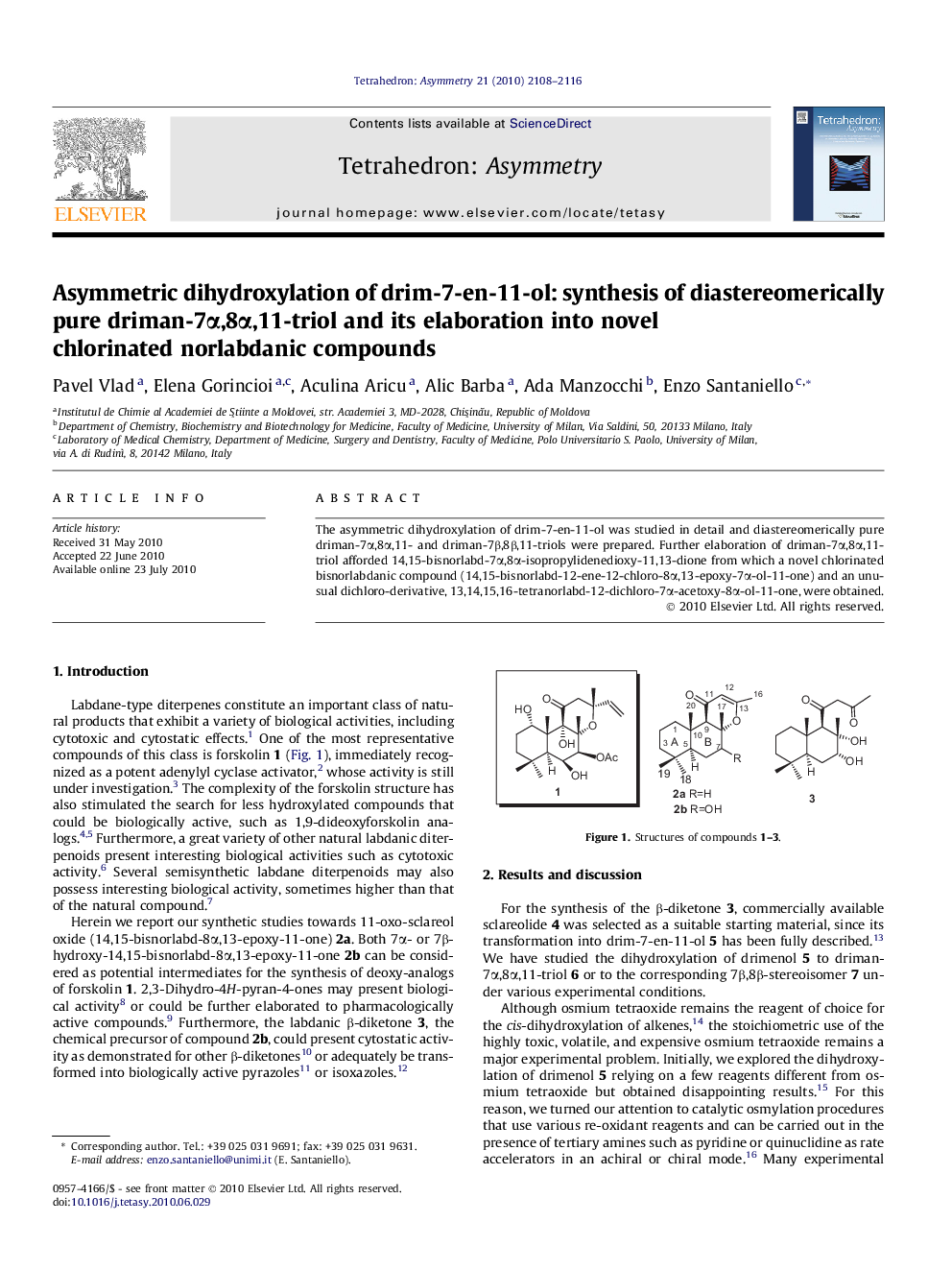
| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346581 | Tetrahedron: Asymmetry | 2010 | 9 Pages |
The asymmetric dihydroxylation of drim-7-en-11-ol was studied in detail and diastereomerically pure driman-7α,8α,11- and driman-7β,8β,11-triols were prepared. Further elaboration of driman-7α,8α,11-triol afforded 14,15-bisnorlabd-7α,8α-isopropylidenedioxy-11,13-dione from which a novel chlorinated bisnorlabdanic compound (14,15-bisnorlabd-12-ene-12-chloro-8α,13-epoxy-7α-ol-11-one) and an unusual dichloro-derivative, 13,14,15,16-tetranorlabd-12-dichloro-7α-acetoxy-8α-ol-11-one, were obtained.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(1S,2S,3R,4aS,8aS)-2,5,5,8a-Tetramethyl-1-(hydroxymethyl)-2,3-decahydronaphthalenediol (drimane-7α,8α,11-triol)C15H28O3[α]D20=-22 (c 0.25, chloroform)Source of chirality: asymmetric dihydroxylation (AD-mix-β) of drim-7-en-11-olAbsolute configuration: (1S,2S,3R,4aS,8aS)
(1S,2R,3S,4aS,8aS)-2,5,5,8a-Tetramethyl-1-(hydroxymethyl)-2,3-decahydronaphthalenediol (drimane-7β,8β,11-triol)C15H28O3[α]D20=+1.4 (c 1.0, chloroform)Source of chirality: asymmetric dihydroxylation (AD-mix-α) of drim-7-en-11-olAbsolute configuration: (1S,2R,3S,4aS,8aS)
(4aR,5R,6aS,10aS,10bR)-1H-Naphtho[2,1-b]pyran, 4a,5,6,6a,7,8,9,10,10a,10b-decahydro-3,4a,7,7,10a-pentamethyl-5-hydroxy-2-chloro-1-oneC21H36O3Cl[α]D25=+44 (c 0.66, chloroform)Source of chirality: synthetic transformation of driman-7α,8α,11-triol
(1S,2S,3R,4aS,8aS)-2,5,5,8a-tetramethyl-1-(2,2-dichloroacetyl)-2,3-decahydronaphthalenediol,3-acetateC18H28O4Cl2[α]D25=-75.9[α]D25=-75.9 (c 1.0, dichloromethane)Source of chirality: synthetic transformation of driman-7α,8α,11-triol