| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346627 | Tetrahedron: Asymmetry | 2015 | 6 Pages |
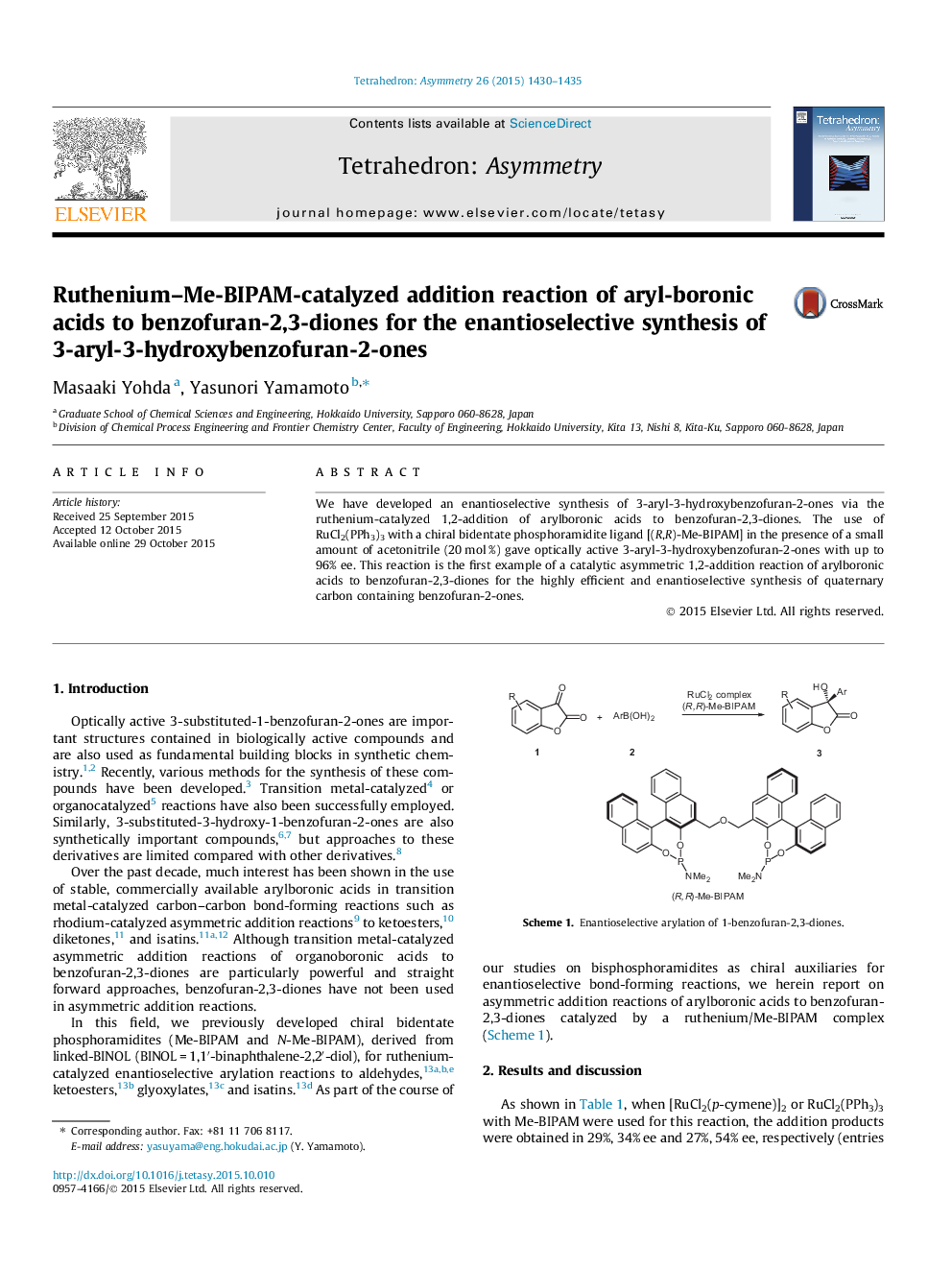
We have developed an enantioselective synthesis of 3-aryl-3-hydroxybenzofuran-2-ones via the ruthenium-catalyzed 1,2-addition of arylboronic acids to benzofuran-2,3-diones. The use of RuCl2(PPh3)3 with a chiral bidentate phosphoramidite ligand [(R,R)-Me-BIPAM] in the presence of a small amount of acetonitrile (20 mol %) gave optically active 3-aryl-3-hydroxybenzofuran-2-ones with up to 96% ee. This reaction is the first example of a catalytic asymmetric 1,2-addition reaction of arylboronic acids to benzofuran-2,3-diones for the highly efficient and enantioselective synthesis of quaternary carbon containing benzofuran-2-ones.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(3S)-3-Hydroxy-4,6-dimethyl-3-phenyl-2,3-dihydro-1-benzofuran-2-oneC16H14O3Ee = 93% from HPLC[α]D22 = +49.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-Hydroxy-4,6-dimethyl-3-(4-phenoxyphenyl)-2,3-dihydro-1-benzofuran-2-oneC22H18O4Ee = 91% from HPLC[α]D23 = +68.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-Hydroxy-4,6-dimethyl-3-(4-methylsulfanylphenyl)-2,3-dihydro-1-benzofuran-2-oneC17H16O3SEe = 90% from HPLC[α]D23 = +114.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-Hydroxy-4,6-dimethyl-3-(4-methylphenyl)-2,3-dihydro-1-benzofuran-2-oneC17H16O3Ee = 91% from HPLC[α]D23 = +83.3 (c 0.54, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-Hydroxy-4,6-dimethyl-3-(4-phenylphenyl)-2,3-dihydro-1-benzofuran-2-oneC22H18O3Ee = 91% from HPLC[α]D23 = +69.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-(4-Chlorophenyl)-3-hydroxy-4,6-dimethyl-2,3-dihydro-1-benzofuran-2-oneC16H13ClO3Ee = 95% from HPLC[α]D22 = +86.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-(4-Fluorophenyl)-3-hydroxy-4,6-dimethyl-2,3-dihydro-1-benzofuran-2-oneC16H13FO3Ee = 94% from HPLC[α]D23 = +69.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-Hydroxy-4,6-dimethyl-3-(4-(trifluoromethyl)phenyl)-2,3-dihydro-1-benzofuran-2-oneC17H13F3O3Ee = 94% from HPLC[α]D23 = +57.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-(4-Ethenylphenyl)-3-hydroxy-4,6-dimethyl-2,3-dihydro-1-benzofuran-2-oneC18H16O3Ee = 94% from HPLC[α]D23 = +63.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-Hydroxy-3-(naphthalen-2-yl)-4,6-dimethyl-2,3-dihydro-1-benzofuran-2-oneC20H16O3Ee = 96% from HPLC[α]D23 = +37.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-(2H-1,3-Benzodioxol-5-yl)-3-hydroxy-4,6-dimethyl-2,3-dihydro-1-benzofuran-2-oneC17H14O5Ee = 88% from HPLC[α]D23 = +100.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-Hydroxy-3-(3,5-dimethylphenyl)-4,6-dimethyl-2,3-dihydro-1-benzofuran-2-oneC18H18O3Ee = 92% from HPLC[α]D23 = +63.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-3-Hydroxy-3-(3-chlorophenyl)-4,6-dimethyl-2,3-dihydro-1-benzofuran-2-oneC16H13ClO3Ee = 94% from HPLC[α]D23 = +64.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3R)-3-(2-Fluorophenyl)-3-hydroxy-4,6-dimethyl-2,3-dihydro-1-benzofuran-2-oneC16H13FO3Ee = 87% from HPLC[α]D24 = −135.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (R)
(3S)-3-Hydroxy-3-phenyl-4,7-dimethyl--2,3-dihydro-1-benzofuran-2-oneC16H14O3Ee = 93% from HPLC[α]D23 = +57.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)
(3S)-5-Bromo-3-hydroxy-4,6-dimethyl-3-phenyl-2,3-dihydro-1-benzofuran-2-oneC16H13BrO3Ee = 94% from HPLC[α]D23 = +86.0 (c 0.50, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (S)
(3S)-3-Hydroxy-4-methyl-3-phenyl-2,3-dihydro-1-benzofuran-2-oneC15H12O3Ee = 92% from HPLC[α]D23 = +29.0 (c 0.50, CHCl3)Source of chirality: Asymmetric arylationAbsolute configuration: (S)