| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346648 | Tetrahedron: Asymmetry | 2010 | 4 Pages |
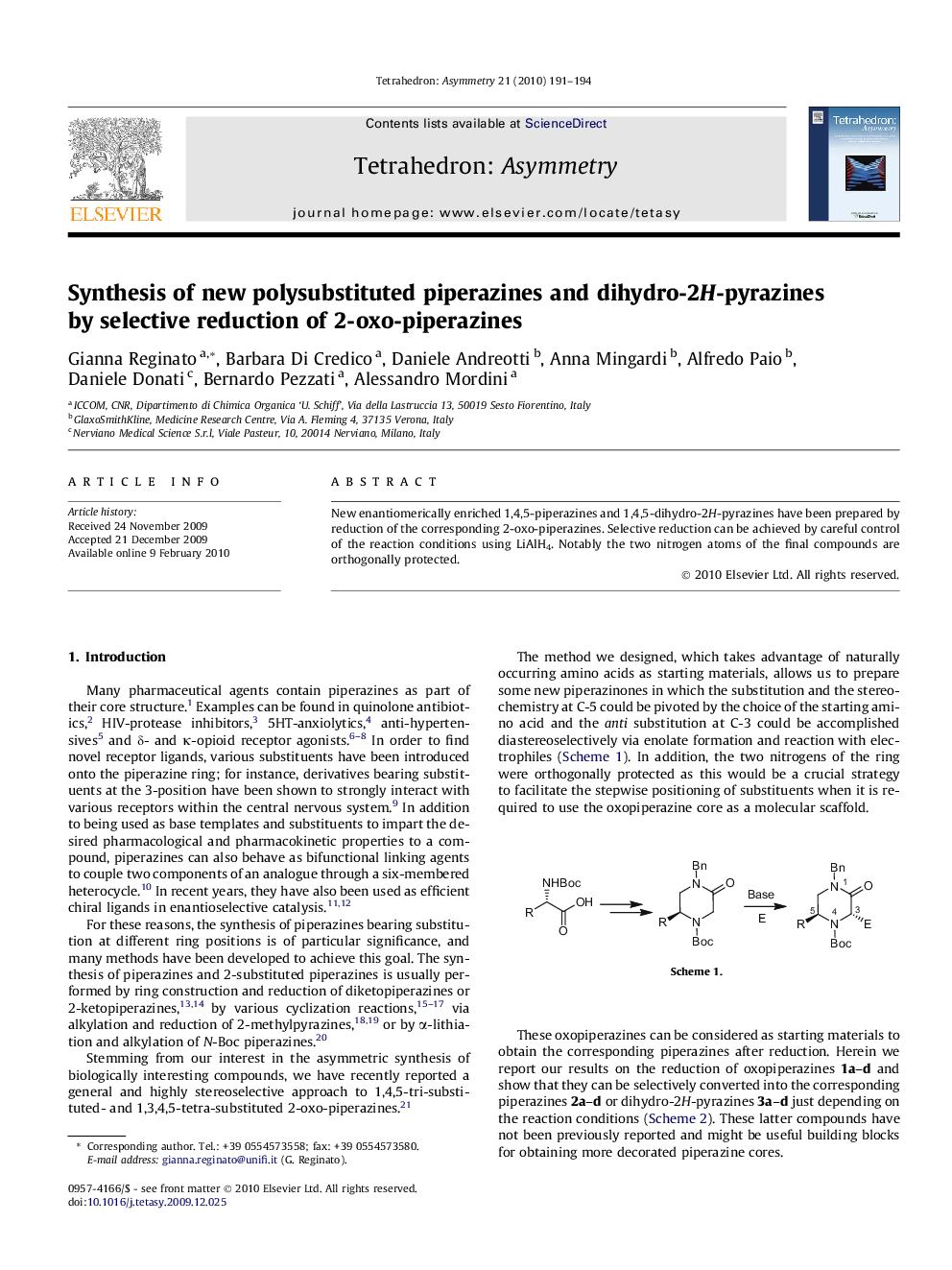
New enantiomerically enriched 1,4,5-piperazines and 1,4,5-dihydro-2H-pyrazines have been prepared by reduction of the corresponding 2-oxo-piperazines. Selective reduction can be achieved by careful control of the reaction conditions using LiAlH4. Notably the two nitrogen atoms of the final compounds are orthogonally protected.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-tert-Butyl-2-methyl-4-benzyl-3,4-dihydropyrazine-1(2H)-carboxylateC17H24N2O2[α]D28=-78.0 (c 0.85, CHCl3)Source of chirality: l-alanineAbsolute configuration: (S)
(S)-tert-Butyl-2-isopropyl-4-benzyl-3,4-dihydropyrazine-1(2H)-carboxylateC19H28N2O2[α]D26=-39.6 (c 1.05, CHCl3)Source of chirality: l-valineAbsolute configuration: (S)
(S)-tert-Butyl-2-isobutyl-4-benzyl-3,4-dihydropyrazine-1(2H)-carboxylateC20H30N2O2[α]D26=-17.7 (c 1.42, CHCl3)Source of chirality: l-leucineAbsolute configuration: (S)
(S)-tert-Butyl-2-[4-(benzyloxy)benzyl]-4-benzyl-3,4-dihydropyrazine-1(2H)-carboxylateC30H34N2O3[α]D26=-86.0 (c 0.6, CHCl3)Source of chirality: l-tyrosineAbsolute configuration: (S)
(S)-tert-Butyl-2-methyl-4-benzylpiperazine-1-carboxylateC17H26N2O2[α]D28=+21.3 (c 0.5, CHCl3)Source of chirality: l-alanineAbsolute configuration: (S)
(S)-tert-Butyl-2-isopropyl-4-benzylpiperazine-1-carboxylateC19H30N2O2[α]D28=+31.0 (c 1.25, CHCl3)Source of chirality: l-valineAbsolute configuration: (S)
(S)-tert-Butyl-2-isobutyl-4-benzylpiperazine-1-carboxylateC20H32N2O2[α]D25=+27.1 (c 1.19, CHCl3)Source of chirality: l-leucineAbsolute configuration: (S)
(S)-tert-Butyl-2-[4-(benzyloxy)benzyl]-4-benzylpiperazine-1-carboxylateC30H36N2O3[α]D27=+1.0 (c 0.5, CHCl3)Source of chirality: l-tyrosineAbsolute configuration: (S)