| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346687 | Tetrahedron: Asymmetry | 2010 | 6 Pages |
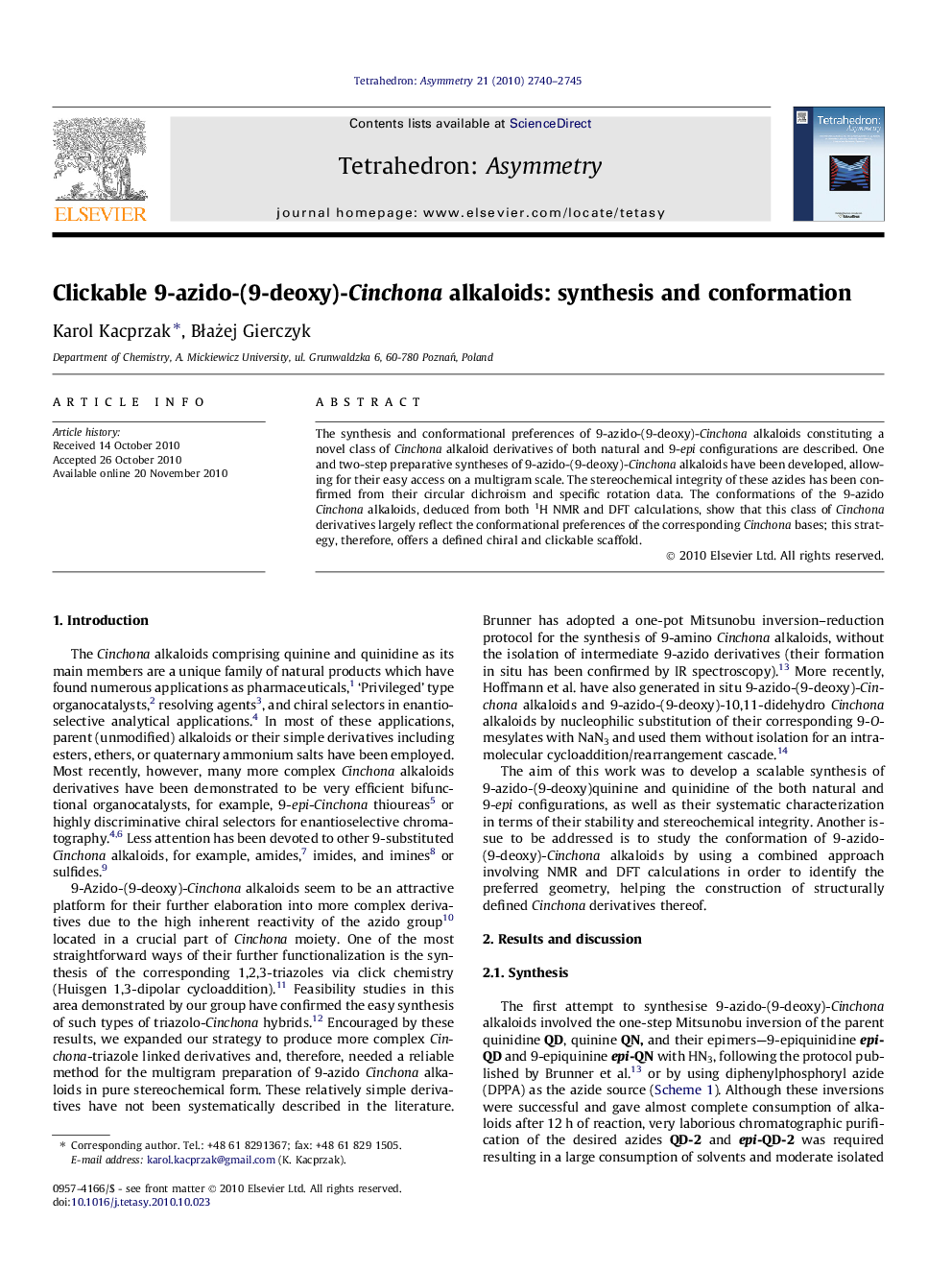
The synthesis and conformational preferences of 9-azido-(9-deoxy)-Cinchona alkaloids constituting a novel class of Cinchona alkaloid derivatives of both natural and 9-epi configurations are described. One and two-step preparative syntheses of 9-azido-(9-deoxy)-Cinchona alkaloids have been developed, allowing for their easy access on a multigram scale. The stereochemical integrity of these azides has been confirmed from their circular dichroism and specific rotation data. The conformations of the 9-azido Cinchona alkaloids, deduced from both 1H NMR and DFT calculations, show that this class of Cinchona derivatives largely reflect the conformational preferences of the corresponding Cinchona bases; this strategy, therefore, offers a defined chiral and clickable scaffold.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(8S,9R)-9-Azido-(9-deoxy)quinineC20H23N5OEe >99%[α]D = −28.2 (c 1.1, CHCl3)Source of chirality: quinineAbsolute configuration: (3R,4S,8S,9R)
(8S,9S)-9-Azido-(9-deoxy)epiquinineC20H23N5OEe >99%[α]D = +77.5 (c 1.0, CHCl3)Source of chirality: quinineAbsolute configuration: (3R,4S,8S,9S)
(8R,9S)-9-Azido-(9-deoxy)quinidineC20H23N5OEe >99%[α]D = +28.0 (c 1.0, CHCl3)Source of chirality: quinidineAbsolute configuration: (3R,4S,8R,9S)
(8R,9R)-9-Azido-(9-deoxy)epiquinidineC20H23N5OEe >99%[α]D = +55.7 (c 1.0, CHCl3)Source of chirality: quinidineAbsolute configuration: (3R,4S,8R,9R)