| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346727 | Tetrahedron: Asymmetry | 2015 | 8 Pages |
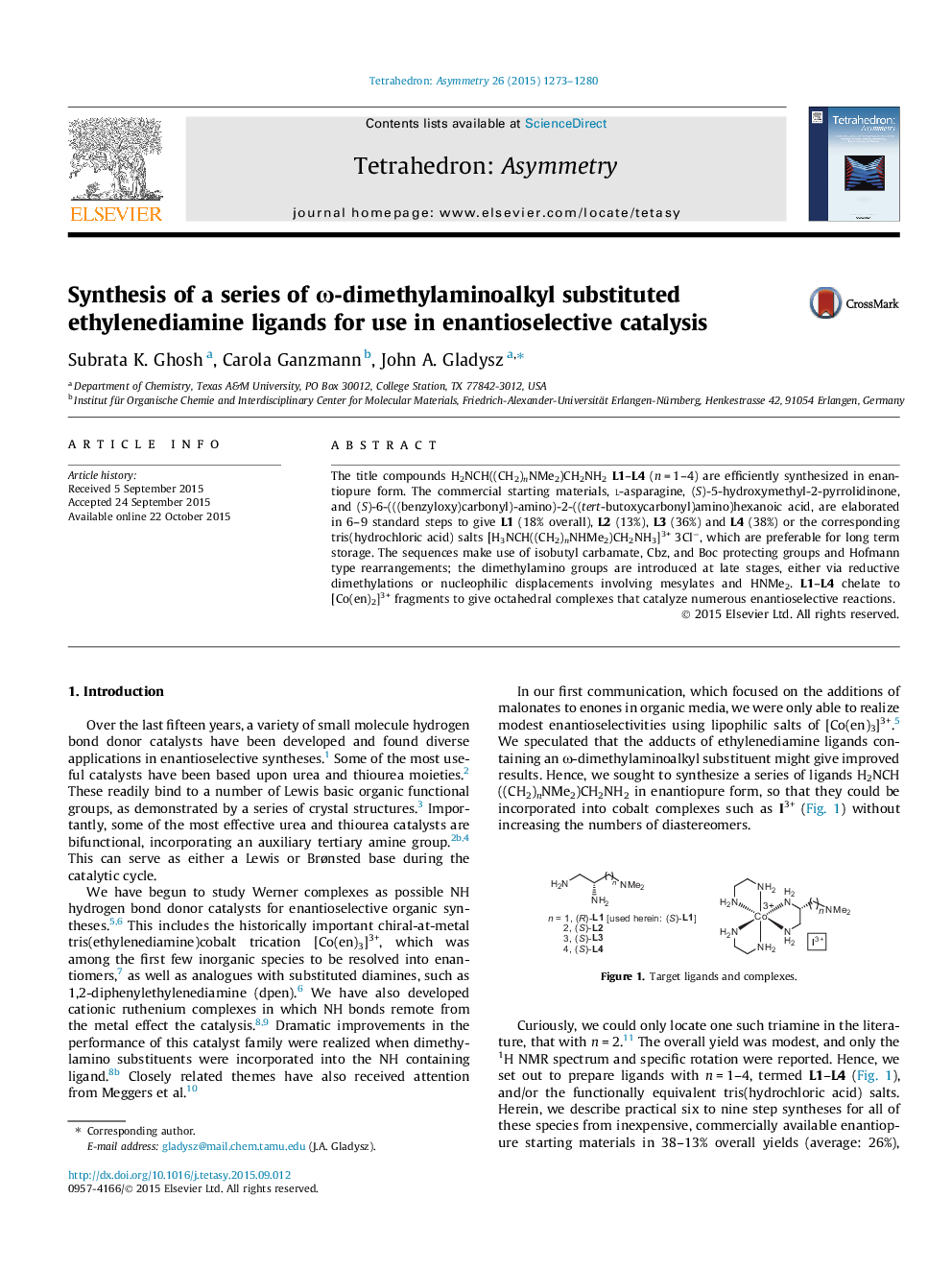
The title compounds H2NCH((CH2)nNMe2)CH2NH2L1–L4 (n = 1–4) are efficiently synthesized in enantiopure form. The commercial starting materials, l-asparagine, (S)-5-hydroxymethyl-2-pyrrolidinone, and (S)-6-(((benzyloxy)carbonyl)-amino)-2-((tert-butoxycarbonyl)amino)hexanoic acid, are elaborated in 6–9 standard steps to give L1 (18% overall), L2 (13%), L3 (36%) and L4 (38%) or the corresponding tris(hydrochloric acid) salts [H3NCH((CH2)nNHMe2)CH2NH3]3+ 3Cl−, which are preferable for long term storage. The sequences make use of isobutyl carbamate, Cbz, and Boc protecting groups and Hofmann type rearrangements; the dimethylamino groups are introduced at late stages, either via reductive dimethylations or nucleophilic displacements involving mesylates and HNMe2. L1–L4 chelate to [Co(en)2]3+ fragments to give octahedral complexes that catalyze numerous enantioselective reactions.
Graphical abstractThe title compounds are prepared in six to nine steps and 13–38% overall yields from the three reasonably priced enantiopure precursors shown above.Figure optionsDownload full-size imageDownload as PowerPoint slide
(S)-N1,N1-Dimethyl-N2-benzyloxycarbonyl-N3-tert-butyloxycarbonyl-N1,N1-dimethyl-propane-1,2,3-triamineC18H29N3O4[α]24589 = −2.6 ± 0.2 (c 0.125, CH3OH)Initial source of chirality: l-AsparagineAbsolute configuration: (S)
(S)-N2-Benzyloxycarbonyl-N1,N1-dimethylpropane-1,2,3-triamineC13H21N3O2[α]24589 = −5.3 ± 0.2 (c 1.15, CH3OH)Initial source of chirality: l-AsparagineAbsolute configuration: (S)
(S)-N1,N1-Dimethylpropane-1,2,3-triamineC5H15N3[α]24589 = −9.3 ± 0.1 (c 0.108, CH3OH)Initial source of chirality: l-AsparagineAbsolute configuration: (S)
Tris(hydrochloric acid) salt of (S)-N1,N1-dimethylpropane-1,2,3-triamineC5H18Cl3N3[α]24589 = −8.7 ± 0.4 (c 0.116, H2O)Initial source of chirality: l-AsparagineAbsolute configuration: (S)