| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346774 | Tetrahedron: Asymmetry | 2010 | 4 Pages |
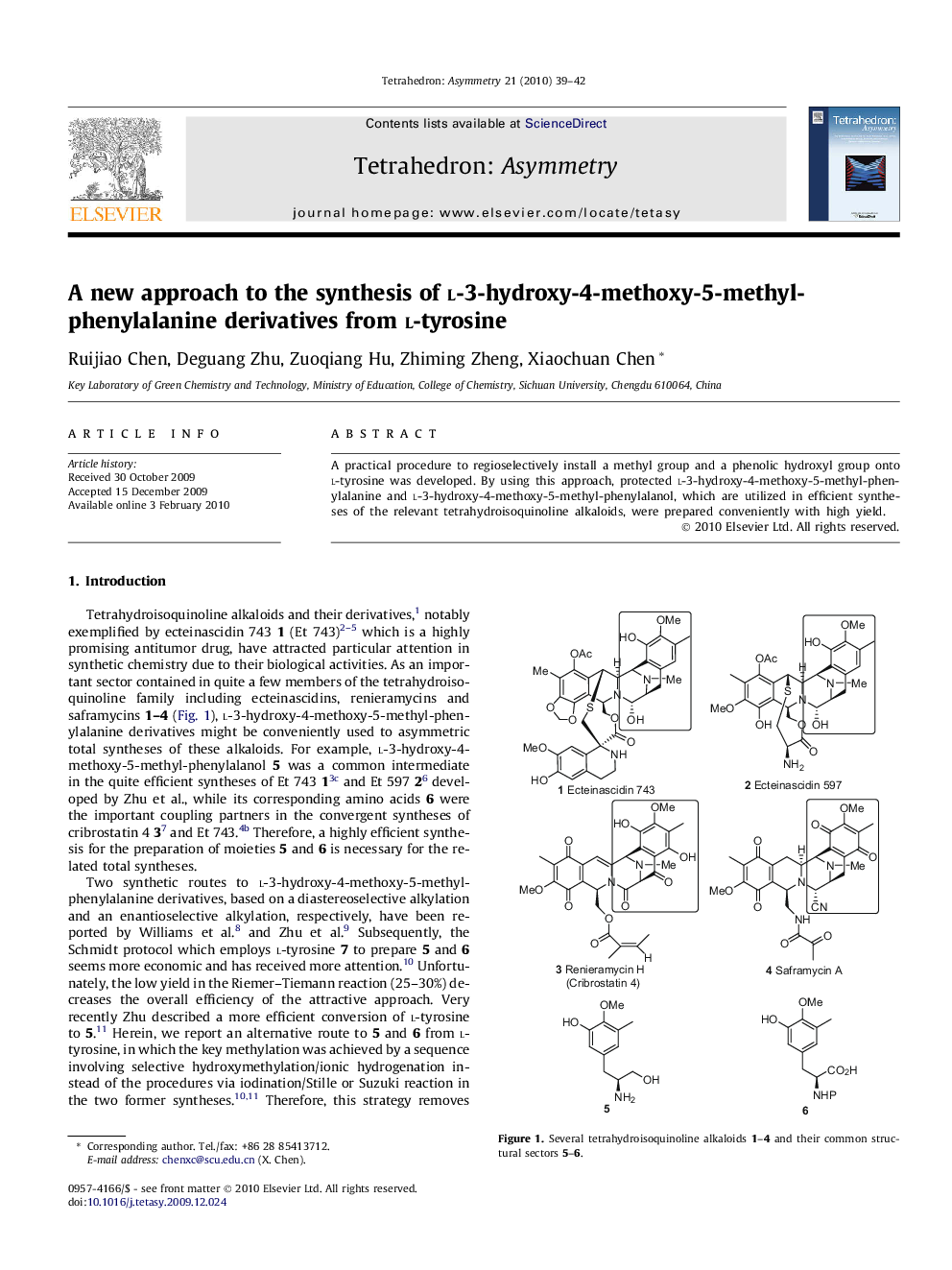
A practical procedure to regioselectively install a methyl group and a phenolic hydroxyl group onto l-tyrosine was developed. By using this approach, protected l-3-hydroxy-4-methoxy-5-methyl-phenylalanine and l-3-hydroxy-4-methoxy-5-methyl-phenylalanol, which are utilized in efficient syntheses of the relevant tetrahydroisoquinoline alkaloids, were prepared conveniently with high yield.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
l-N-Benzyloxycarbonyl-3-methyl-4-methoxy-phenylalanine methyl esterC20H23NO5[α]D26=+49 (c 0.99, CHCl3)Source of chirality: l-tyrosineAbsolute configuration: (S)
l-N-Benzyloxycarbonyl-3-formyl-4-methoxy-5-methyl-phenylalanine methyl esterC21H23NO6[α]D27=+63 (c 1.1, CHCl3)Source of chirality: l-tyrosineAbsolute configuration: (S)
l-N-Benzyloxycarbonyl-3-hydroxy-4-methoxy-5-methyl-phenylalanolC19H23NO5[α]D27=-23 (c 1.1, CHCl3)Source of chirality: l-tyrosineAbsolute configuration: (S)
l-N-Benzyloxycarbonyl-3-hydroxy-4-methoxy-5-methyl-phenylalanineC19H21NO6[α]D27=+41 (c 1.1, CHCl3)Source of chirality: l-tyrosineAbsolute configuration: (S)
l-3-Hydroxy-4-methoxy-5-methyl-phenylalanolC11H17NO3[α]D27=-17 (c 0.99, CHCl3)Source of chirality: l-tyrosineAbsolute configuration: (S)