| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346833 | Tetrahedron: Asymmetry | 2010 | 9 Pages |
Starting from the racemic ethyl 3-hydroxy-3-(10-alkyl-10H-phenothiazin-3-yl)propanoates as substrates, a multienzymatic procedure was developed for the efficient synthesis of the corresponding highly enantiomerically enriched (R)- and (S)-3-heteroaryl-3-hydroxypropanoic acids.
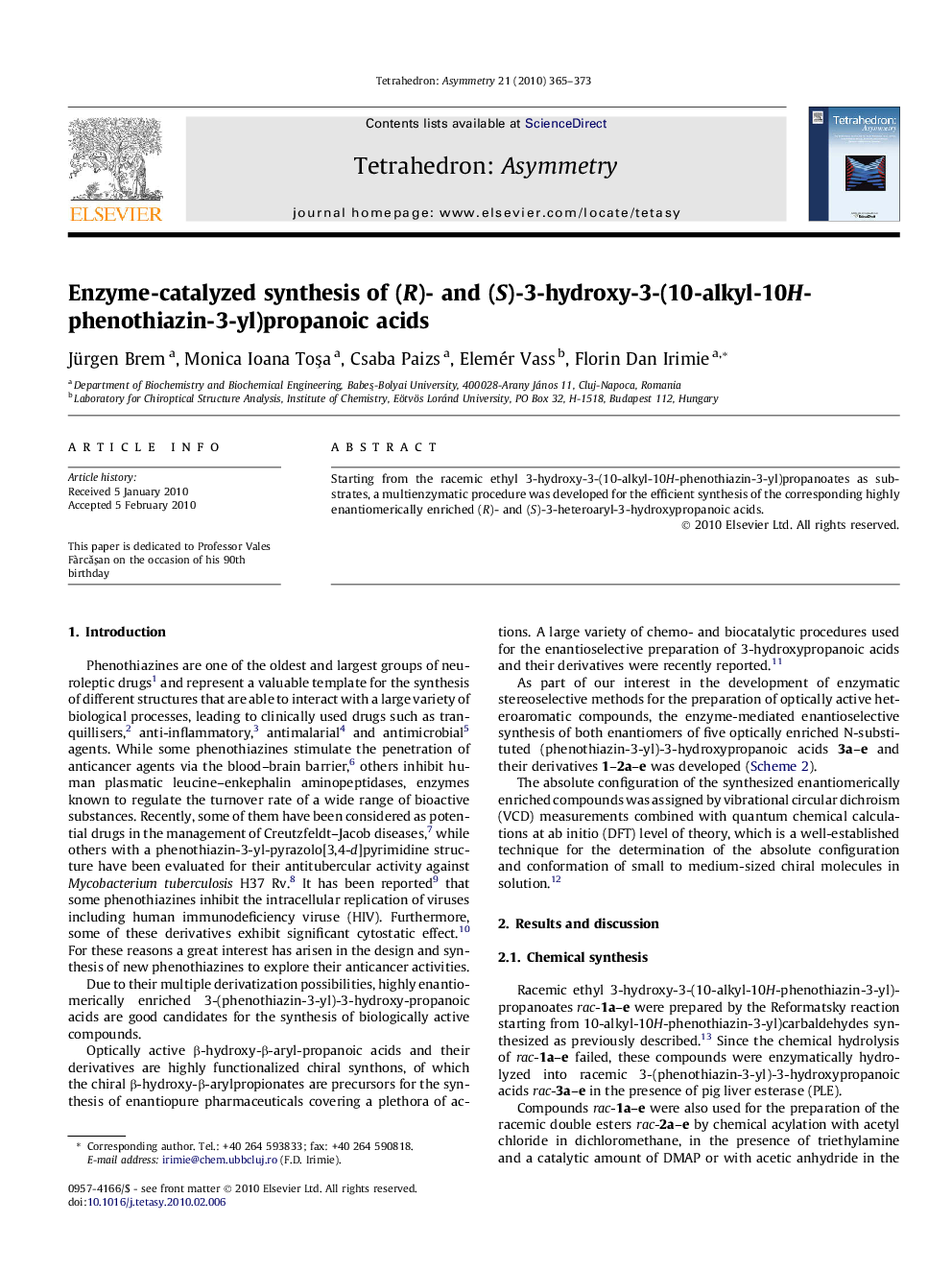
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-3-Hydroxy-3-(10-methyl-10H-phenothiazin-3-yl)propanoic acidC16H15NO3SEe = 95.5% on Astec Chirobiotic R HPLC column[α]D20=-23.6 (c 1, DMF)Absolute configuration: (S)
(S)-3-Hydroxy-3-(10-ethyl-10H-phenothiazin-3-yl)propanoic acidC17H17NO3SEe = 98% on Astec Chirobiotic R HPLC column[α]D20=-19.5 (c 1, DMF)Absolute configuration: (S)
(S)-3-Hydroxy-3-(10-propyl-10H-phenothiazin-3-yl)propanoic acidC18H19NO3SEe = 92% on Astec Chirobiotic R HPLC column[α]D20=-28 (c 1, DMF)Absolute configuration: (S)
(S)-3-Hydroxy-3-(10-butyl-10H-phenothiazin-3-yl)propanoic acidC19H21NO3SEe = 96% on Astec Chirobiotic R HPLC column[α]D20=-27.1 (c 1, DMF)Absolute configuration: (S)
(S)-3-Hydroxy-3-(10-pentyl-10H-phenothiazin-3-yl)propanoic acidC20H23NO3SEe = 95% on Astec Chirobiotic R HPLC column[α]D20=-27.2 (c 1, DMF)Absolute configuration: (S)