| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346846 | Tetrahedron: Asymmetry | 2015 | 8 Pages |
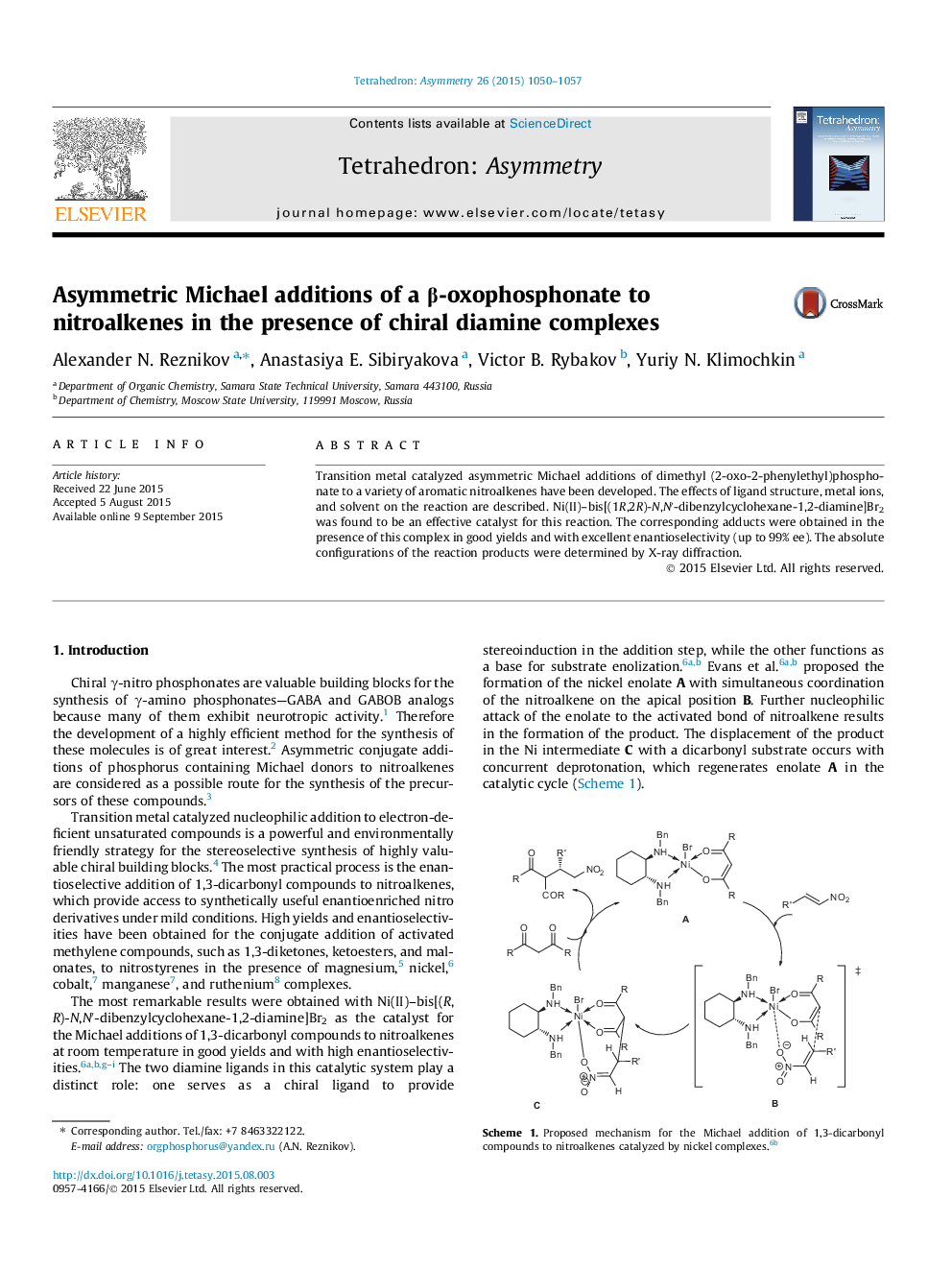
Transition metal catalyzed asymmetric Michael additions of dimethyl (2-oxo-2-phenylethyl)phosphonate to a variety of aromatic nitroalkenes have been developed. The effects of ligand structure, metal ions, and solvent on the reaction are described. Ni(II)–bis[(1R,2R)-N,N′-dibenzylcyclohexane-1,2-diamine]Br2 was found to be an effective catalyst for this reaction. The corresponding adducts were obtained in the presence of this complex in good yields and with excellent enantioselectivity (up to 99% ee). The absolute configurations of the reaction products were determined by X-ray diffraction.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Dimethyl [(2R,3S)-4-nitro-1-oxo-1,3-diphenylbut-2-yl]phosphonateC18H20NO6P[α]D20 = −38.4 (с 1.0, CHCl3)Absolute configuration: (2R,3S)Source of chirality: asymmetric catalysis
Dimethyl [(2R,3S)-3-(4-chlorophenyl)-4-nitro-1-oxo-1-phenylbut-2-yl]phosphonateC18H19ClNO6P[α]D20 = −34.7 (с 2.5, CH2Cl2)Absolute configuration: (2R,3S)Source of chirality: asymmetric catalysis
Dimethyl [(3S)-3-(2-chlorophenyl)-4-nitro-1-oxo-1-phenylbut-2-yl]phosphonateC18H19ClNO6P[α]D20 = −47.3 (с 2.5, CHCl3)Absolute configuration: (3S)Source of chirality: asymmetric catalysis
Dimethyl [(2R,3S)-3-(2-methoxyphenyl)-4-nitro-1-oxo-1-phenylbut-2-yl]phosphonateC19H22NO7P[α]D20 = −44.7 (с 2.5, CHCl3)Absolute configuration: (2R,3S)Source of chirality: asymmetric catalysis
Dimethyl [(2S,3R)-3-(3-methoxyphenyl)-4-nitro-1-oxo-1-phenylbut-2-yl]phosphonateC19H22NO7P[α]D20 = +42.4 (с 2.5, CH2Cl2)Absolute configuration: (2S,3R)Source of chirality: asymmetric catalysis
Dimethyl [(2R,3S)-3-(4-methoxyphenyl)-4-nitro-1-oxo-1-phenylbut-2-yl]phosphonateC19H22NO7P[α]D20 = −42.4 (с 2.5, CH2Cl2)Absolute configuration: (2R,3S)Source of chirality: asymmetric catalysis
Dimethyl [(2R,3S)-3-(3,4,5-trimethoxyphenyl)-4-nitro-1-oxo-1-phenylbut-2-yl]phosphonateC21H26NO9P[α]D20 = −35.6 (с 2.5, CHCl3)Absolute configuration: (2R,3S)Source of chirality: asymmetric catalysis