| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1346966 | Tetrahedron: Asymmetry | 2009 | 4 Pages |
Abstract
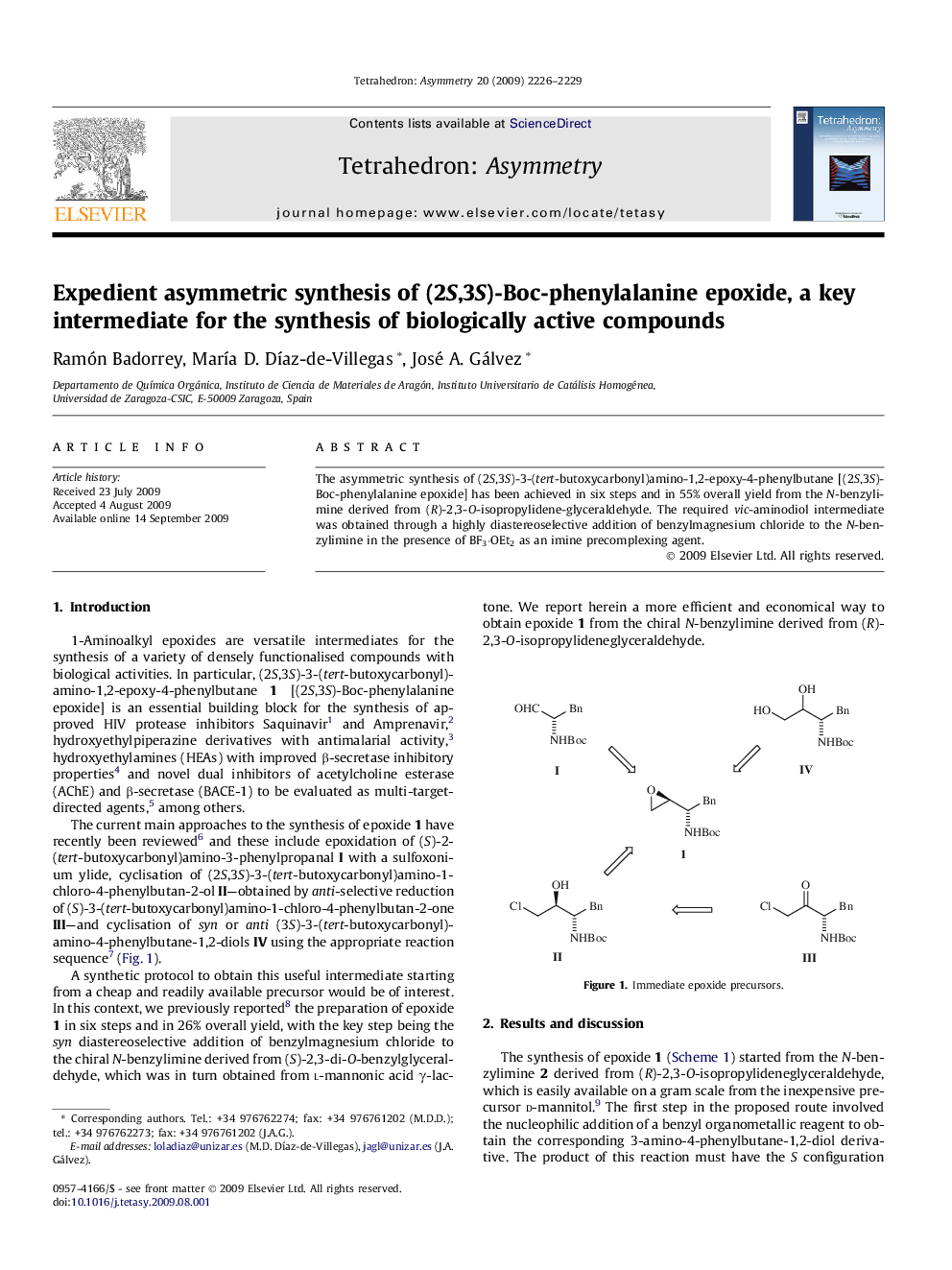
The asymmetric synthesis of (2S,3S)-3-(tert-butoxycarbonyl)amino-1,2-epoxy-4-phenylbutane [(2S,3S)-Boc-phenylalanine epoxide] has been achieved in six steps and in 55% overall yield from the N-benzylimine derived from (R)-2,3-O-isopropylidene-glyceraldehyde. The required vic-aminodiol intermediate was obtained through a highly diastereoselective addition of benzylmagnesium chloride to the N-benzylimine in the presence of BF3·OEt2 as an imine precomplexing agent.
Related Topics
Physical Sciences and Engineering
Chemistry
Inorganic Chemistry
Authors
Ramón Badorrey, MarÃa D. DÃaz-de-Villegas, José A. Gálvez,