| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1347115 | Tetrahedron: Asymmetry | 2014 | 5 Pages |
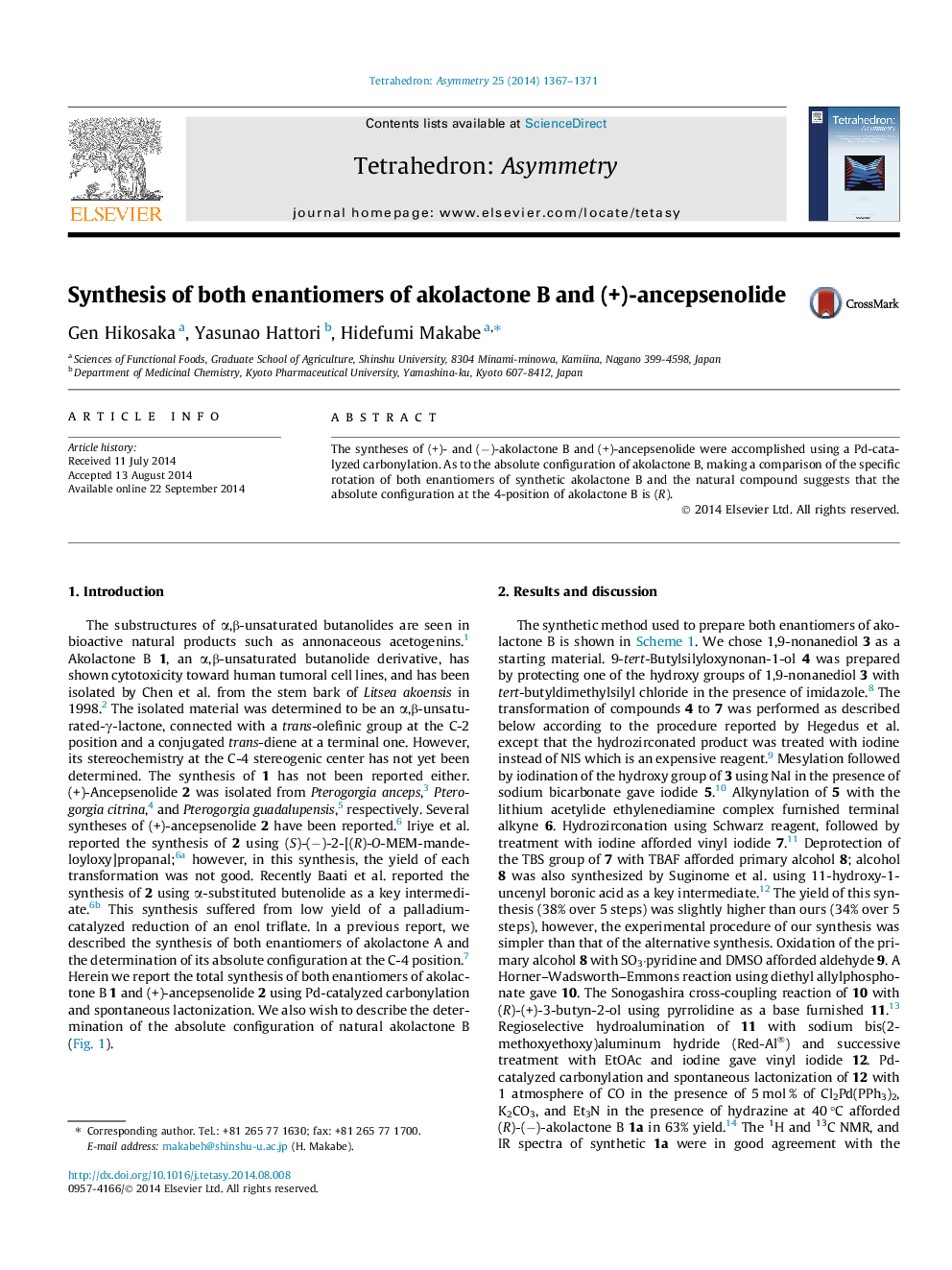
The syntheses of (+)- and (−)-akolactone B and (+)-ancepsenolide were accomplished using a Pd-catalyzed carbonylation. As to the absolute configuration of akolactone B, making a comparison of the specific rotation of both enantiomers of synthetic akolactone B and the natural compound suggests that the absolute configuration at the 4-position of akolactone B is (R).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(R)-(−)-Akolactone BC19H28O2[α]D18 = 38.1 (c 1.10, CHCl3)Source of chirality (R)-3-butyn-2-olAbsolute configuration: (2R)
(+)-AncepsenolideC22H34O4[α]D18 = +47.1 (c 1.28, CHCl3)Source of chirality (S)-3-butyn-2-olAbsolute configuration: (2S, 19S)
(2R,5E,17E)-Octadeca-5,15,17-trien-3-yn-2-olC18H28O[α]D19 = +15.9 (c 1.23, CHCl3)Source of chirality (R)-3-butyn-2-olAbsolute configuration: (2R)
(2R,3Z,5E,15E)-4-Iodooctadeca-3,5,15,17-tetraen-2-olC18H29IO[α]D19 = +8.6 (c 1.69, CHCl3)Source of chirality (R)-3-butyn-2-olAbsolute configuration: (2R)
(2S,19S)-2,19-Bis-(tert-butyldimethylsilyoxy)eicosa-3,17-diyneC32H62O2Si2[α]D18 = 5.4 (c 1.44, CHCl3)Source of chirality (S)-3-butyn-2-olAbsolute configuration: (2S, 19S)
(2S,19S)-Eicosa-3,17-diyne-2,19-diolC20H34O2[α]D21 = 26.2 (c 0.635, CHCl3)Source of chirality (S)-3-butyn-2-olAbsolute configuration: (2S, 19S)
(2S,19S)-4,17-Diiodoeicosa-3,17-diene-2,19-diolC20H36I2O2[α]D18 = 0.03 (c 1.25, CHCl3)Source of chirality (S)-3-butyn-2-olAbsolute configuration: (2S, 19S)