| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1347334 | Tetrahedron: Asymmetry | 2009 | 7 Pages |
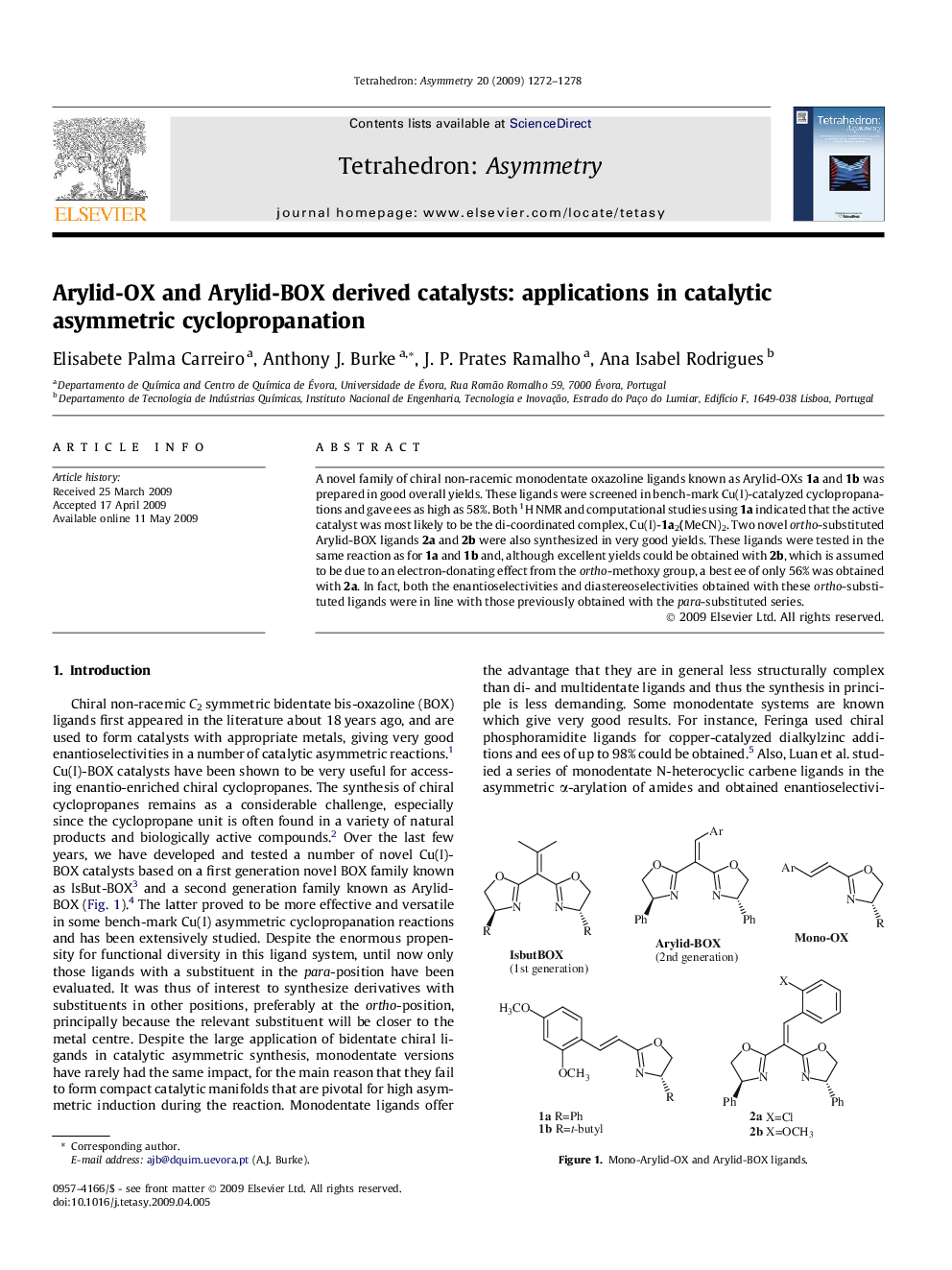
A novel family of chiral non-racemic monodentate oxazoline ligands known as Arylid-OXs 1a and 1b was prepared in good overall yields. These ligands were screened in bench-mark Cu(I)-catalyzed cyclopropanations and gave ees as high as 58%. Both 1H NMR and computational studies using 1a indicated that the active catalyst was most likely to be the di-coordinated complex, Cu(I)-1a2(MeCN)2. Two novel ortho-substituted Arylid-BOX ligands 2a and 2b were also synthesized in very good yields. These ligands were tested in the same reaction as for 1a and 1b and, although excellent yields could be obtained with 2b, which is assumed to be due to an electron-donating effect from the ortho-methoxy group, a best ee of only 56% was obtained with 2a. In fact, both the enantioselectivities and diastereoselectivities obtained with these ortho-substituted ligands were in line with those previously obtained with the para-substituted series.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(+)-(S)-trans-2-(2,4-Dimethoxyphenyl)-1-(4-phenyloxazoline-2-yl)etheneC19H19NO3[α]D22=+7.7 (c 0.57, CHCl3)Source of chirality: starting materialAbsolute configuration: (S)
(−)-(S)-trans-2-(2,4-Dimethoxyphenyl)-1-(4-tert-butyloxazoline-2-yl)etheneC17H23NO3[α]D21=-38.3 (c 1.44, CHCl3)Source of chirality: starting materialAbsolute configuration: (S)
(+)-Bis[(S)-4-phenyloxazoline-2-yl]-2-(2-chlorophenyl)etheneC26H21ClN2O2[α]D20=+50.3 (c 1.89, CHCl3)Source of chirality: starting materialAbsolute configuration: (S)
(+)-Bis[(S)-4-phenyloxazoline-2-yl]-2-(2-methoxyphenyl)etheneC27H24N2O3[α]D20=+137.8 (c 0.94, CHCl3)Source of chirality: starting materialAbsolute configuration: (S)