| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1347388 | Tetrahedron: Asymmetry | 2008 | 6 Pages |
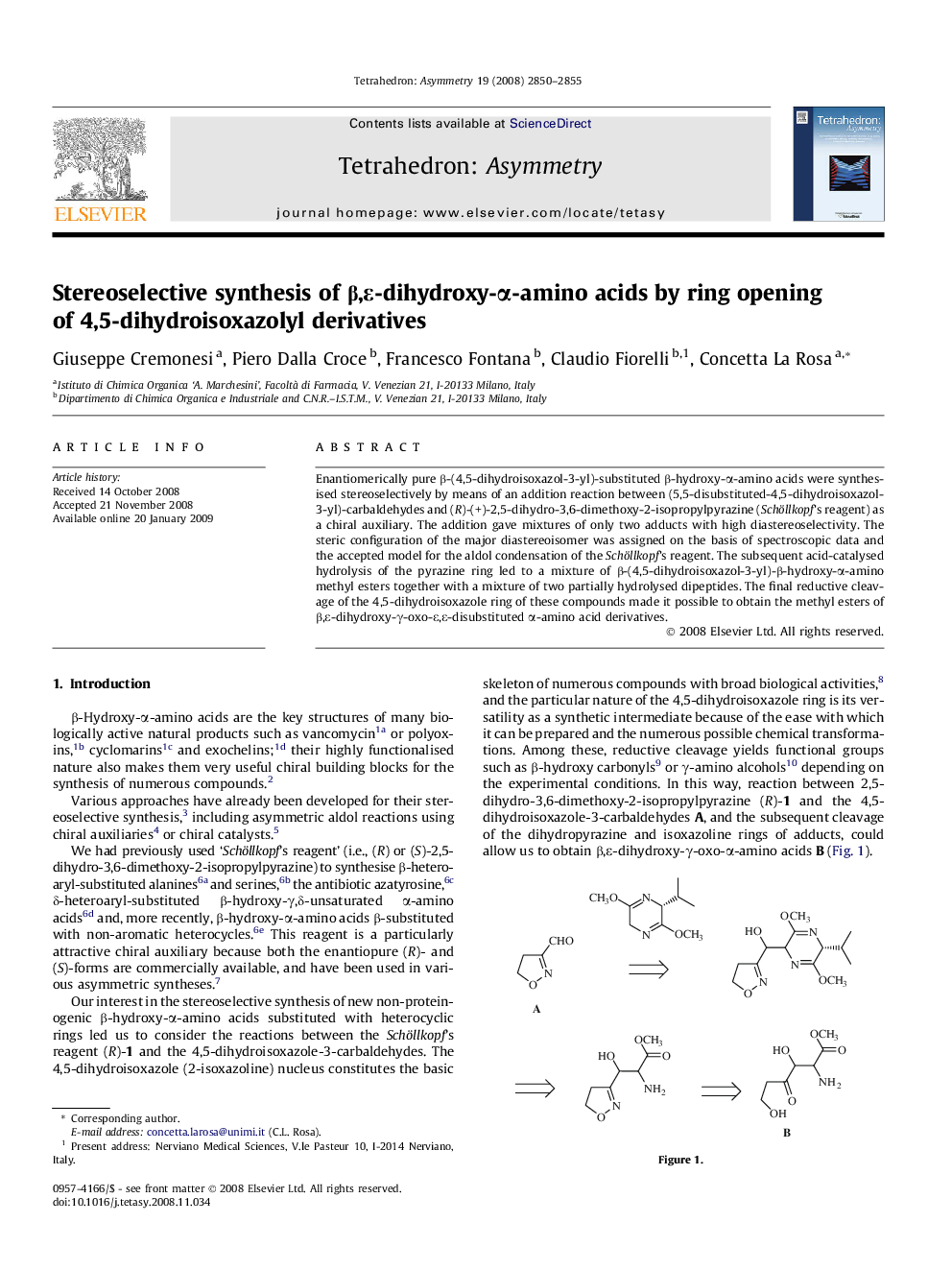
Enantiomerically pure β-(4,5-dihydroisoxazol-3-yl)-substituted β-hydroxy-α-amino acids were synthesised stereoselectively by means of an addition reaction between (5,5-disubstituted-4,5-dihydroisoxazol-3-yl)-carbaldehydes and (R)-(+)-2,5-dihydro-3,6-dimethoxy-2-isopropylpyrazine (Schöllkopf’s reagent) as a chiral auxiliary. The addition gave mixtures of only two adducts with high diastereoselectivity. The steric configuration of the major diastereoisomer was assigned on the basis of spectroscopic data and the accepted model for the aldol condensation of the Schöllkopf’s reagent. The subsequent acid-catalysed hydrolysis of the pyrazine ring led to a mixture of β-(4,5-dihydroisoxazol-3-yl)-β-hydroxy-α-amino methyl esters together with a mixture of two partially hydrolysed dipeptides. The final reductive cleavage of the 4,5-dihydroisoxazole ring of these compounds made it possible to obtain the methyl esters of β,ε-dihydroxy-γ-oxo-ε,ε-disubstituted α-amino acid derivatives.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-(5,5-Diphenyl-4,5-dihydro-isoxazol-3-yl)-[(2S,5R)-5-isopropyl-3,6-dimethoxy-2,5-dihydro-pyrazin-2-yl]-methanolC25H29N3O4Dr = >99% (NMR)[α]D20=-14.4 (c 0.60, CHCl3)Source of chirality: Schöllkopf’s reagentAbsolute configuration: (2S,5R,1′S)
(S)-[(2S,5R)-5-Isopropyl-3,6-dimethoxy-2,5-dihydro-pyrazin-2-yl]-(1-oxa-2-aza-spiro[4.5]dec-2-en-3-yl)-methanolC18H29N3O4Dr = >99% (NMR)[α]D20=-45.0 (c 0.58, CHCl3)Source of chirality: Schöllkopf’s reagentAbsolute configuration: (2S,5R,1′S)
(S)-(5,5-Dimethyl-4,5-dihydro-isoxazol-3-yl)-[(2S,5R)-5-isopropyl-3,6-dimethoxy-2,5-dihydro-pyrazin-2-yl]-methanolC15H25N3O4Dr = >99% (NMR)[α]D20=-57.2 (c 0.84, CHCl3)Source of chirality: Schöllkopf’s reagentAbsolute configuration: (2S,5R,1′S)
(2S,3S)-2-Amino-3-(5,5-diphenyl-4,5-dihydro-isoxazol-3-yl)-3-hydroxy-propionic acid methyl esterC19H20N2O4Dr = >99% (NMR)[α]D20=+8.0 (c 0.37, CHCl3)Source of chirality: Schöllkopf’s reagentAbsolute configuration: (2S,3S)
(2S,3S,2′R)-2-(2-Amino-3-methyl-butyrylamino)-3-(5,5-diphenyl-4,5-dihydro-isoxazol-3-yl)-3-hydroxy-propionic acid methyl esterC24H29N3O5Dr = >99% (NMR)[α]D20=-1.8 (c 1.13, CHCl3)Source of chirality: Schöllkopf’s reagentAbsolute configuration: (2S,3S,2′R)
(2S,3S,2′R)-2-(2-Amino-3-methyl-butyrylamino)-3,6-dihydroxy-6-methyl-4-oxo-eptanoic methyl esterC14H26N2O6Dr = >99% (NMR)[α]D20=+6.8 (c 0.28, CH3OH)Source of chirality: Schöllkopf’s reagentAbsolute configuration: (2S,3S,2′R)
(2S,3S)-2-Amino-3-hydroxy-3-(1-oxa-2-aza-spiro[4.5]dec-2-en-3-yl)-propionic acid methyl esterC12H20N2O4Dr = >99% (NMR)[α]D20=+15.1 (c 0.22, CH3OH)Source of chirality: Schöllkopf’s reagentAbsolute configuration: (2S,3S)
(2S,3S,2′R)-2-(2-Amino-3-methyl-butyrylamino)-3-hydroxy-3-(1-oxa-2-aza-spiro[4.5]dec-2-en-3-yl)-propionic acid methyl esterC17H29N3O5Dr = >99% (NMR)[α]D20=-1.7 (c 0.76, CHCl3)Source of chirality: Schöllkopf’s reagentAbsolute configuration: (2S,3S,2′R)
(2S,3S)-2-Amino-3-(5,5-dimethyl-4,5-dihydro-isoxazol-3-yl)-3-hydroxy-propionic acid methyl esterC9H16N2O4Dr = >99% (NMR)[α]D20=+4.7 (c 0.32, CHCl3)Source of chirality: Schöllkopf’s reagentAbsolute configuration: (2S,3S)
(2S,3S,2′R)-2-(2-Amino-3-methyl-butyrylamino)-3-(5,5-dimethyl-4,5-dihydro-isoxazol-3-yl)-3-hydroxy-propionic acid methyl esterC14H25N3O5Dr = >99% (NMR)[α]D20=+10.9 (c 1.27, CHCl3)Source of chirality: Schöllkopf’s reagentAbsolute configuration: (2S,3SR)
(2S,3S,2′R)-2-(2-Amino-3-methyl-butyrylamino)-3-hydroxy-5-(1-hydroxycyclohexyl)-4-oxo-propionic acid methyl esterC17H30N2O6Dr = >99% (NMR)[α]D20=+57.25 (c 0.29, CHCl3)Source of chirality: Schöllkopf’s reagentAbsolute configuration: (2S,3SR)