| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1347469 | Tetrahedron: Asymmetry | 2009 | 9 Pages |
The cis- and trans-4-benzylparaconic acids and their ethyl esters were synthesized with high enantiomeric excess by hydrolysis of the corresponding diastereomeric lactonic esters using α-chymotrypsin. Thus, at low conversion values, cis- and trans-4-benzyl-5-oxo-3-tetrahydrofurancarboxylic acids were separately isolated with 99% ee and 92% ee, respectively. Both ethyl ester diastereomers were also obtained in enantiopure form. The absolute configuration of the trans-lactonic acid was assigned by 1H NMR analysis of its ester derivatives with both enantiomers of 1-(9-anthryl)-2,2,2-trifluoroethanol, while that of the cis-lactonic acid was assigned by means of X-ray analysis of a crystalline derivative. The circular dichroism curves of the products obtained are also reported.
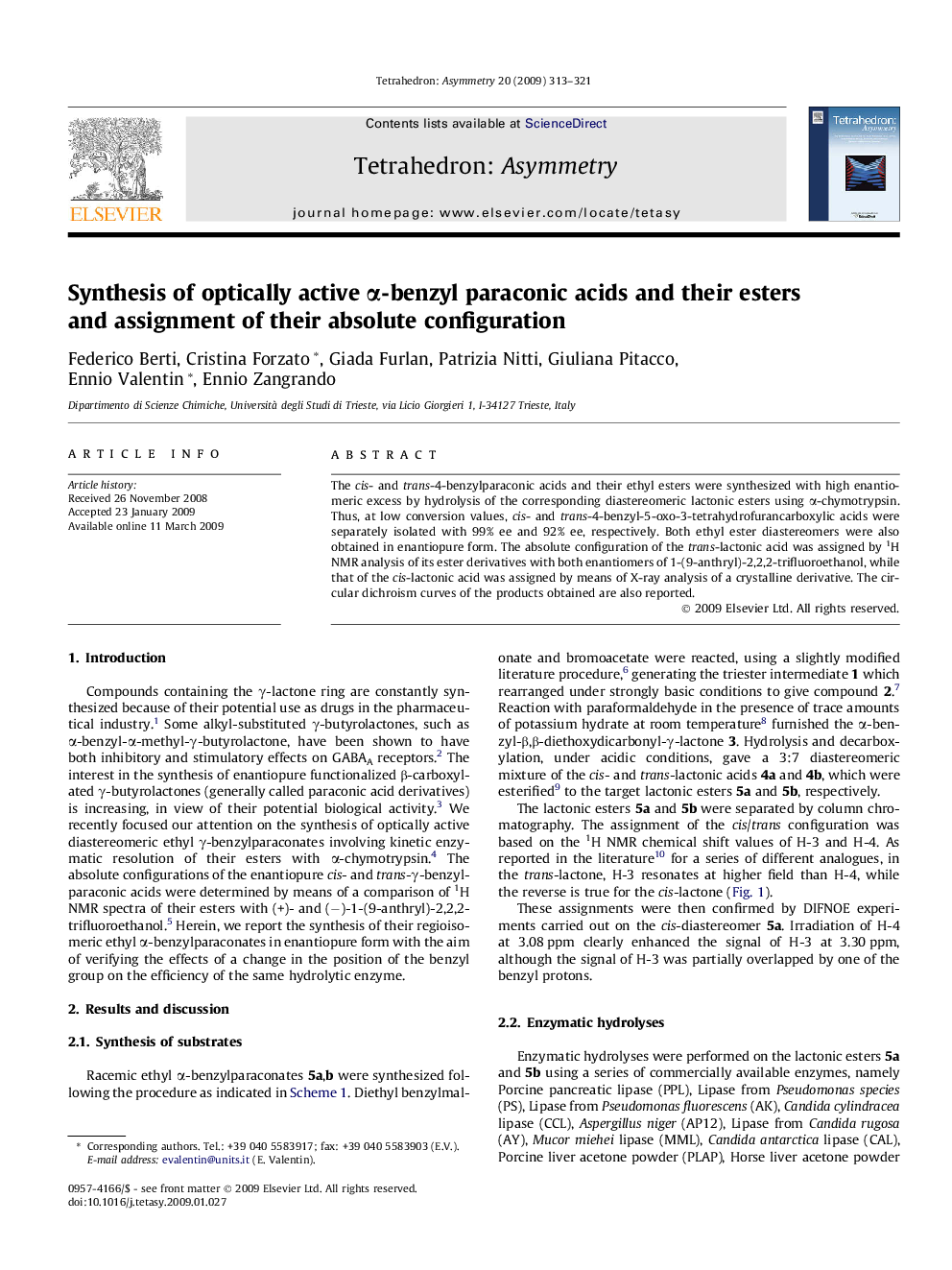
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
trans-(−)-(3S,4S)-4-Benzyl-5-oxo-3-tetrahydrofurancarboxylic acidC12H12O4Ee = 92% (by chiral HRGC)[α]D25=-29.2 (c 0.24, MeOH)Δε221 = +0.8, Δε263 = +0.03 (MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (3S,4S)
Ethyl trans-(+)-(3R,4R)-4-benzyl-5-oxo-3-tetrahydrofurancarboxylateC14H16O4Ee = 96% (by chiral HRGC)[α]D25=+34.6 (c 0.24, MeOH)Δε221 = −2.6, Δε263 = −0.1 (MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (3R,4R)
Ethyl cis-(+)-(3R,4S)-4-benzyl-5-oxo-3-tetrahydrofurancarboxylateC14H16O4Ee = 94% (by chiral HRGC)[α]D25=+124.8 (c 1.4, MeOH)Δε217 = +5.0, Δε262 = +0.1 (MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (3R,4S)
cis-(−)-(3S,4R)-4-Benzyl-5-oxo-3-tetrahydrofurancarboxylic acidC12H12O4Ee = 99% (by chiral HRGC)[α]D25=-103.0 (c 0.93, MeOH)Δε217 = −3.7, Δε263 = −0.1 (MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (3S,4R)
4-Bromophenacyl cis-(−)-(3S,4R)-4-benzyl-5-oxo-3-tetrahydrofurancarboxylateC20H17O5BrEe = 99%[α]D25=-76.8c 0.5, MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (3S,4R)