| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1347585 | Tetrahedron: Asymmetry | 2013 | 6 Pages |
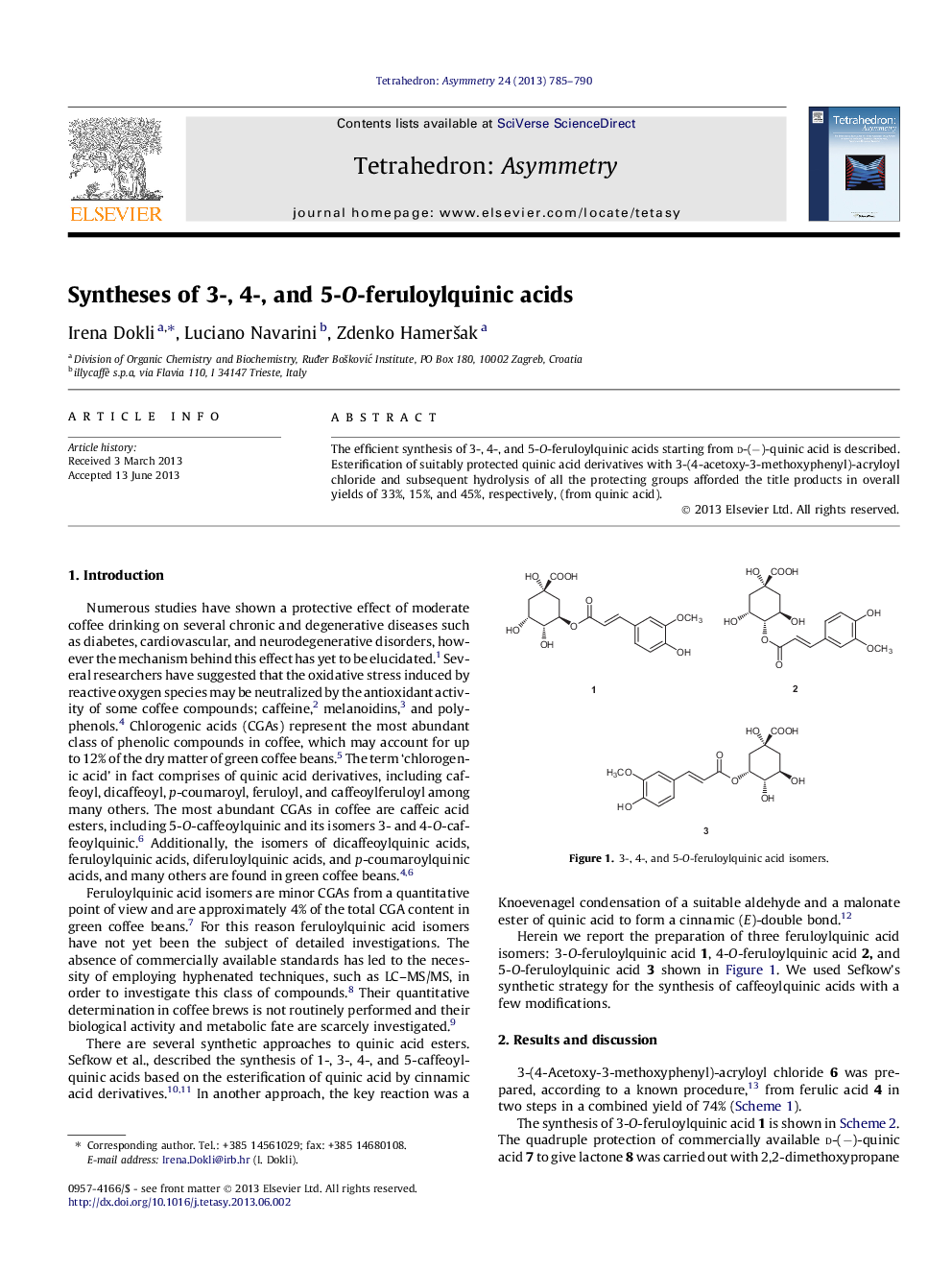
The efficient synthesis of 3-, 4-, and 5-O-feruloylquinic acids starting from d-(−)-quinic acid is described. Esterification of suitably protected quinic acid derivatives with 3-(4-acetoxy-3-methoxyphenyl)-acryloyl chloride and subsequent hydrolysis of all the protecting groups afforded the title products in overall yields of 33%, 15%, and 45%, respectively, (from quinic acid).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(1S,3R,4R,5R)-1,4,5-Trihydroxy-3-[3-(4-hydroxy-3-methoxy-phenyl)-acryloyloxy] cyclohexanecarboxylic acidC17H20O9[α]D25=-35.1 (c 1.14, CH3OH)Source of chirality: d-(−)-quinic acidAbsolute configuration: (1S,3R,4R,5R)
(1S,3R,4S,5R)-1,3,5-Trihydroxy-4-[3-(4-hydroxy-3-methoxy-phenyl)-acryloyloxy] cyclohexanecarboxylic acidC17H20O9[α]D25=-55.7 (c 0.79, CH3OH).Source of chirality: d-(−)-quinic acidAbsolute configuration: (1S,3R,4S,5R)
(1R,3R,4S,5R)-1,3,4-Trihydroxy-5-[3-(4-hydroxy-3-methoxy-phenyl)-acryloyloxy] cyclohexanecarboxylic acidC17H20O9[α]D25=+10.9 (c 0.64, CH3OH)Source of chirality: d-(−)-quinic acidAbsolute configuration: (1R,3R,4S,5R)