| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1347682 | Tetrahedron: Asymmetry | 2013 | 10 Pages |
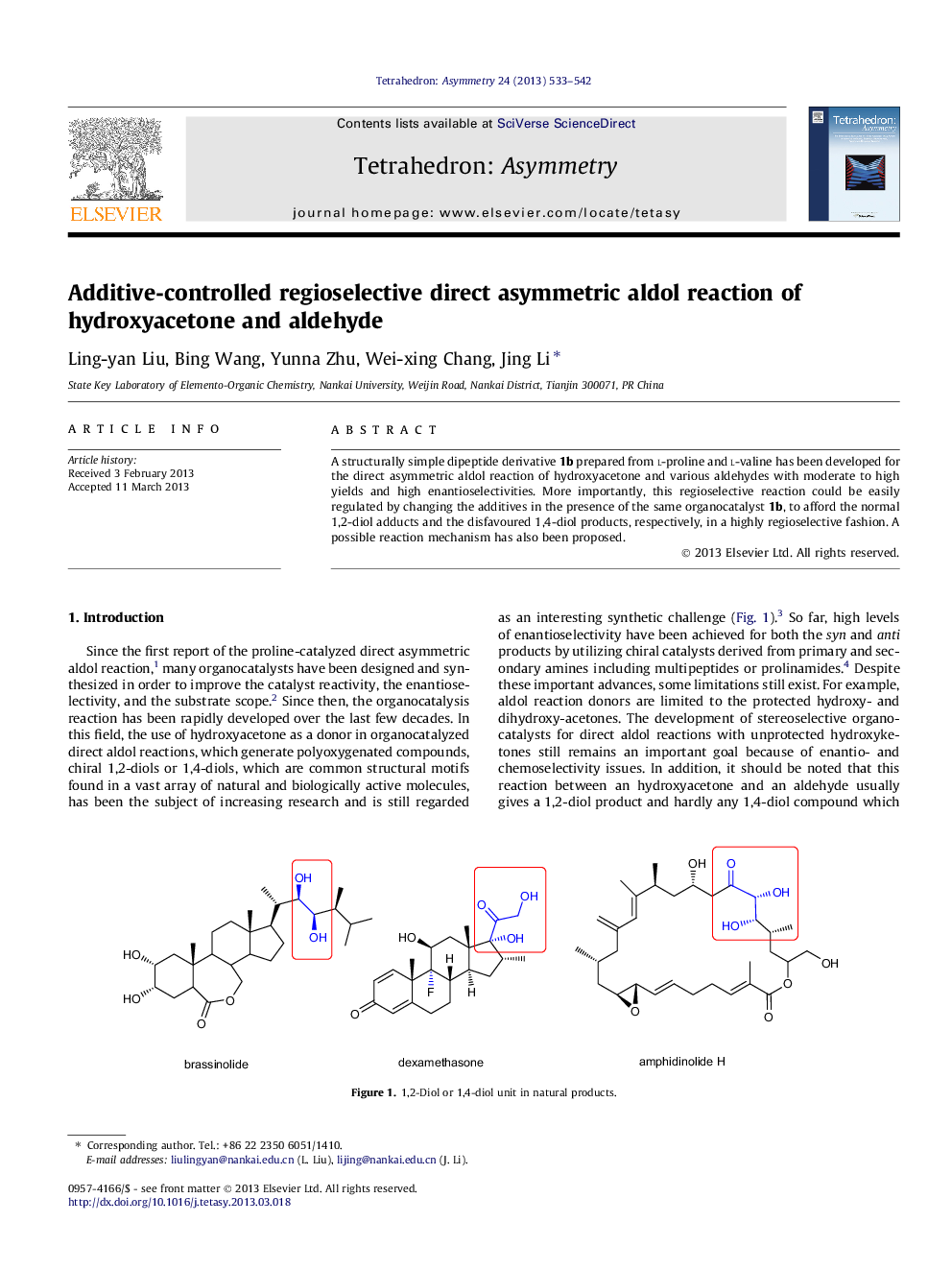
A structurally simple dipeptide derivative 1b prepared from l-proline and l-valine has been developed for the direct asymmetric aldol reaction of hydroxyacetone and various aldehydes with moderate to high yields and high enantioselectivities. More importantly, this regioselective reaction could be easily regulated by changing the additives in the presence of the same organocatalyst 1b, to afford the normal 1,2-diol adducts and the disfavoured 1,4-diol products, respectively, in a highly regioselective fashion. A possible reaction mechanism has also been proposed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-tert-Butyl 2-((S)-1-(methoxycarbonyl)-2-methylpropyl-carbamoyl)pyrrolidine-1-carboxylateC16H28N2O5[α]D20=-102.0 (c 1.0, EtOH)Source of chirality: the precursorAbsolute configuration: (S,S)
(S)-Methyl 2-((S)-pyrrolidine-2-carbothioamido)-3-methylbutanoateC11H20N2O2S[α]D20=-53.6 (c 1.0, CH2Cl2)Source of chirality: the precursorAbsolute configuration: (S,S)