| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1347738 | Tetrahedron: Asymmetry | 2008 | 10 Pages |
The stereoselective synthesis of (2-phenylcyclopropyl)methylidene acetals of sugar derivatives from methyl d-glucopyranoside, dodecyl N-acetyl-2-amino-2-deoxy-d-glucopyranoside, 1,2-O-isopropylidene-d-glucofuranose, methyl l-rhamnopyranoside and 1,2-O-isopropylidene-d-xylofuranose, as a chiral auxiliary, is described. The cyclopropanation reaction of the corresponding alkenylidene derivatives with CH2I2/ZnEt2 took place with different stereoselectivity, depending on the configuration on the acetal carbon, the size of the acetal ring, the sugar configuration, the protecting group of the hydroxyl groups of the sugar and the substitution of the unsaturated system. The stereochemistry of the new stereogenic centres was then determined by acid hydrolysis of the cyclopropane moiety of the chiral auxiliary, which was also recovered.
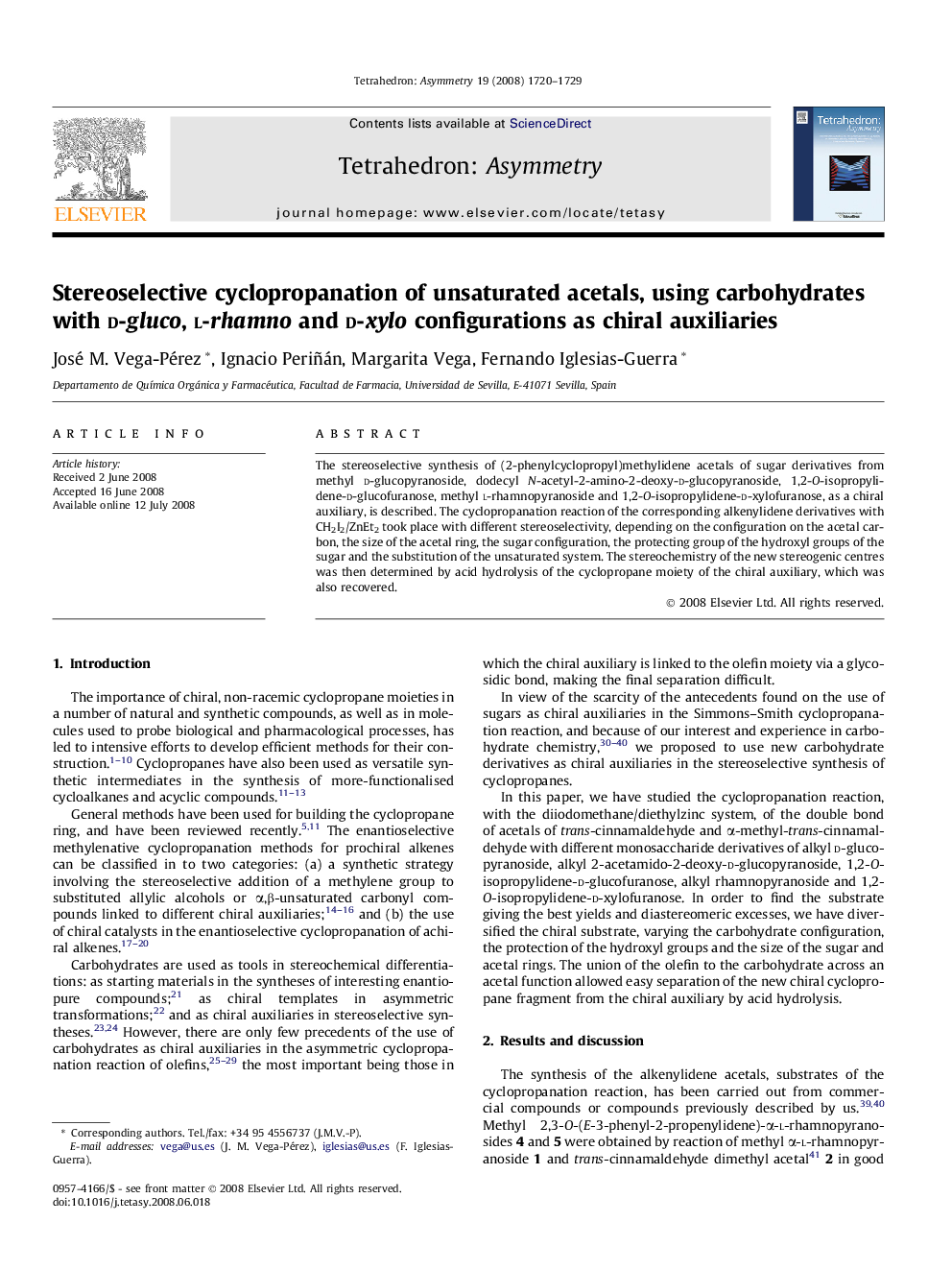
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Methyl 4-O-benzyl-2,3-O-[(S,E)-3-phenyl-2-propenylidene]-α-l-rhamnopyranosideC23H26O5Ee = 100%[α]D25=-63.2 (c 1.2, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 2,3-O-(S,E)-, α-l-rhamno
Methyl 4-O-benzyl-2,3-O-[(R,E)-3-phenyl-2-propenylidene]-α-l-rhamnopyranosideC23H26O5Ee = 100%[α]D25=-36.0 (c 0.7, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 2,3-O-(R,E)-, α-l-rhamno
Methyl 4-O-benzyl-2,3-O-[(S,E)-2-methyl-3-phenyl-2-propenylidene]-α-l-rhamnopyranosideC24H28O5Ee = 100%[α]D25=-43.8 (c 1.2, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 2,3-O-(S,E)-, α-l-rhamno
Methyl 4-O-benzyl-2,3-O-[(R,E)-2-methyl-3-phenyl-2-propenylidene]-α-l-rhamnopyranosideC24H28O5Ee = 100%[α]D25=-6.0 (c 0.6, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 2,3-O-(R,E)-, α-l-rhamno
3-O-Benzyl-5,6-O-[(S,E)-3-phenyl-2-propenylidene]-1,2-O-isopropylidene-α-d-glucofuranoseC25H28O6Ee = 100%[α]D25=-4.7 (c 0.8, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 5,6-O-(S,E)-, α-d-glucofuranose
3-O-Benzyl-5,6-O-[(S,E)-2-methyl-3-phenyl-2-propenylidene]-1,2-O-isopropylidene-α-d-glucofuranoseC26H30O6Ee = 100%[α]D25=-10.7 (c 0.8, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 5,6-O-(S,E)-, α-d-glucofuranose
Methyl 2,3-di-O-benzyl-4,6-O-[(R,E)-3-phenyl-2-propenylidene]-α-d-glucopyranosideC30H32O6Ee = 100%[α]D25=+6.8 (c 0.9, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 4,6-O-(R,E)-, α-d-glucopyranose
Methyl 2,3-di-O-benzyl-4,6-O-[(R,E)-2-methyl-3-phenyl-2-propenylidene]-α-d-glucopyranosideC31H34O6Ee = 100%[α]D25=+29.2 (c 1.1, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 4,6-O-(R,E)-, α-d-glucopyranose
Methyl 4-O-benzyl-2,3-O-[(1S,2R,3R)-(2-phenylcyclopropyl)methylidene]-α-l-rhamnopyranosideC24H28O5De = 26%[α]D25=-17.8 (c 1.0, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 2,3-O-(1S,2R,3R)-, α-l-rhamno
Methyl 4-O-benzyl-2,3-O-[(1R,2S,3S)-(2-phenylcyclopropyl)methylidene]-α-l-rhamnopyranosideC24H28O5De = 57%[α]D25=-15.3 (c 1.2, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 2,3-O-(1R,2S,3S)-, α-l-rhamno
1,2-O-Isopropylidene-5,6-O-[(1S,2R,3R)-(2-phenylcyclopropyl)methylidene]-α-d-glucofuranoseC19H24O6De = 49%[α]D25=-6.3 (c 1.1, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 5,6-O-(1S,2R,3R)-, α-d-glucofuranose
3-O-Benzyl-1,2-O-isopropylidene-5,6-O-[(1S,2S,3S)-(2-phenylcyclopropyl)methylidene]-α-d-glucofuranoseC26H30O6De = 4.8%[α]D25=+12.9 (c 1.2, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 5,6-O-(1S,2S,3S)-, α-d-glucofuranose
Methyl 4,6-O-[(1R,2R,3R)-(2-phenylcyclopropyl)methylidene]-α-d-glucopyranosideC17H22O6De = 20%[α]D25=+82.8 (c 1.0, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 4,6-O-(1R,2R,3R)-, α-d-glucopyranose
Methyl 4,6-O-[(1R,2R,3S)-(1-methyl-2-phenylcyclopropyl)methylidene]-α-d-glucopyranosideC18H24O6De = 4.8%[α]D25=+94.0 (c 1.0, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 4,6-O-(1R,2R,3S)-, α-d-glucopyranose
Methyl 2,3-di-O-benzyl-4,6-O-[(1R,2R,3R)-(2- phenylcyclopropyl)methylidene]-α-d-glucopyranosideC31H34O6De = 33%[α]D25=+79.9 (c 0.8, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 4,6-O-(1R,2R,3R)-, α-d-glucopyranose
Methyl 2,3-dibenzyl-4,6-O-[(1R,2R,3S)-(1-methyl-2-phenylcyclopropyl)methylidene]-α-d-glucopyranosideC32H36O6De = 4.8%[α]D25=+31.4 (c 0.9, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 4,6-O-(1R,2R,3S)-, α-d-glucopyranose
1-Dodecyl 2-acetamido-3-O-benzyl-2-deoxy-4,6-O-[(1R,2R,3R)-(2-phenylcyclopropyl)methylidene]-β-d-glucopyranosideC37H53NO6De = 46%[α]D25=+4.0 (c 4.6, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 4,6-O-(1R,2R,3R)-, α-d-glucopyranose
1,2-O-Isopropylidene-3,5-O-[(1S,2R,3R)-(2-phenylcyclopropyl)methylidene]-α-d-xylofuranoseC18H22O5De = 80%[α]D25=-60.4 (c 1.0, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 3,5-O-(1S,2R,3R)-, α-d-xylofuranose
1,2-O-Isopropylidene-3,5-O-[(1S,2R,3S)-(1-methyl-2-phenylcyclopropyl)methylidene]-α-d-xylofuranoseC19H24O5De = 50%[α]D25=-12.9 (c 0.9, CH2Cl2)Source of chirality: asymmetric synthesisAbsolute configuration: 3,5-O-(1S,2R,3S)-, α-d-xylofuranose