| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1347813 | Tetrahedron: Asymmetry | 2013 | 8 Pages |
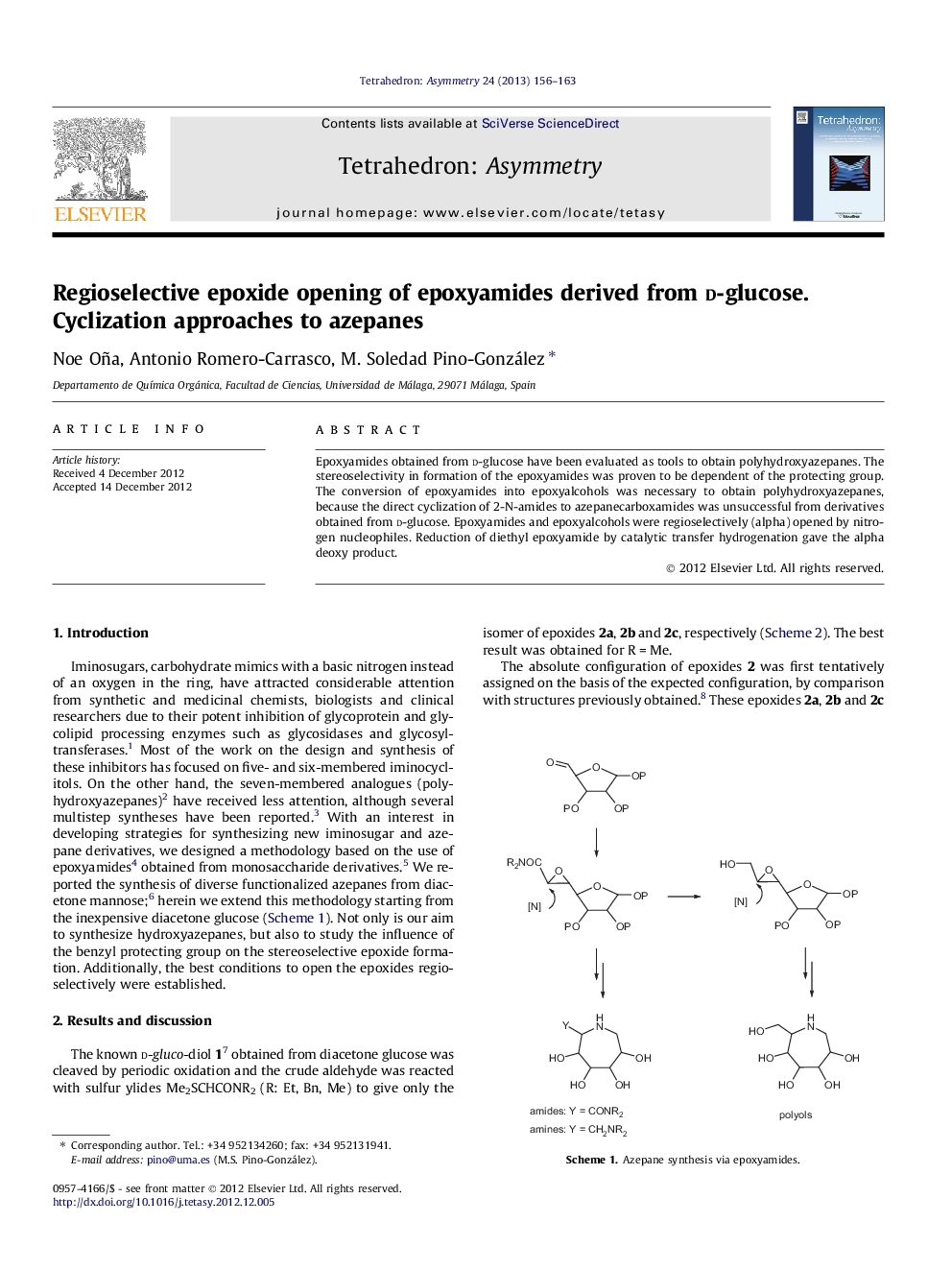
Epoxyamides obtained from d-glucose have been evaluated as tools to obtain polyhydroxyazepanes. The stereoselectivity in formation of the epoxyamides was proven to be dependent of the protecting group. The conversion of epoxyamides into epoxyalcohols was necessary to obtain polyhydroxyazepanes, because the direct cyclization of 2-N-amides to azepanecarboxamides was unsuccessful from derivatives obtained from d-glucose. Epoxyamides and epoxyalcohols were regioselectively (alpha) opened by nitrogen nucleophiles. Reduction of diethyl epoxyamide by catalytic transfer hydrogenation gave the alpha deoxy product.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
N,N-Dimethyl-5,6-anhydro-3-O-benzyl-1,2-O-isopropylidene-l-glycero-α-d-gluco-heptofuranuronamideC19H25NO6[α]D17=-7 (c 5.24, CH2Cl2)Source of chirality: d-glucose and stereoselective synthesis
N,N-Dibenzyl-5,6-anhydro-3-O-benzyl-1,2-O-isopropylidene-l-glycero-α-d-gluco-heptofuranuronamideC31H33NO6[α]D17=+25 (c 0.78, CH2Cl2)Source of chirality: d-glucose and stereoselective synthesis
N,N-Diethyl-5,6-anhydro-3-O-benzyl-1,2-O-isopropylidene-l-glycero-α-d-gluco-heptofuranuronamideC21H29NO6[α]D20=-22 (c 1.20, AcOEt)Source of chirality: d-glucose and stereoselective synthesis
N,N-Dimethyl-6-azido-3-O-benzyl-6-deoxy-1,2-O-isopropylidene-d-glycero-α-d-gluco-heptofuranuronamideC19H26N4O6[α]D24=-1 (c 1.09, CH2Cl2)Source of chirality: d-glucose, stereo- and regioselective syntheses
N,N-Dibenzyl-6-azido-3-O-benzyl-6-deoxy-1,2-O-isopropylidene-d-glycero-α-d-gluco-heptofuranuronamideC31H34N4O6[α]D24=+28 (c 0.50, CH2Cl2)Source of chirality: d-glucose, stereo- and regioselective syntheses
N,N-Dimethyl-6-amino-3-O-benzyl-6-deoxy-1,2-O-isopropylidene-d-glycero-α-d-gluco-heptofuranuronamideC19H28N2O6[α]D20=+1 (c 0.96, AcOEt)Source of chirality: d-glucose, stereo- and regioselective syntheses
N,N-Dibenzyl-6-amine-3-O-benzyl-6-deoxy-1,2-O-isopropylidene-d-glycero-α-d-gluco-heptofuranuronamideC31H36N2O6[α]D20=-27.5 (c 0.92, AcOEt)Source of chirality: d-glucose, stereo- and regioselective syntheses
N,N-Dimethyl-6-aminobenzyl-3-O-benzyl-6-deoxy-1,2-O-isopropylidene-d-glycero-α-d-gluco-heptofuranuronamideC26H34N2O6[α]D17=-7 (c 2.12, CH2Cl2)Source of chirality: d-glucose, stereo- and regioselective syntheses
N,N-Dibenzyl-6-aminobenzyl-3-O-benzyl-6-deoxy-1,2-O-isopropylidene-d-glycero-α-d-gluco-heptofuranuronamideC38H42N2O6[α]D20=-31 (c 0.72, CH2Cl2)Source of chirality: d-glucose, stereo- and regioselective syntheses
N,N-Dimethyl-6-O-acetyl-6-azido-3-O-benzyl-1,2-O-isopropylidene-d-glycero-α-d-gluco-heptofuranuronamideC21H28N4O7[α]D20=-35 (c 1.20, AcOEt)Source of chirality: d-glucose, stereo- and regioselective syntheses
N,N-Dibenzyl-6-O-acetyl-6-azido-3-O-benzyl-1,2-O-isopropylidene-d-glycero-α-d-gluco-heptofuranuronamideC33H36N4O7[α]D20=-17 (c 0.70, MeOH)Source of chirality: d-glucose, stereo- and regioselective syntheses
N,N-Dimethyl-6-(N-benzyloxycarbonylamino)-6-deoxy-1,2-O-isopropylidene-d-glycero-α-d-gluco-heptofuranuronamideC20H28N2O8[α]D17=-15 (c 0.92, CH2Cl2)Source of chirality: d-glucose, stereo- and regioselective syntheses