| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1347845 | Tetrahedron: Asymmetry | 2008 | 4 Pages |
Abstract
A mild and efficient procedure for Michael additions of cyclohexanone to chalcones has been developed. In the presence of amino acid ionic liquid [EMIm][Pro] (200 mol %), cyclohexanone reacted with various chalcones to afford Michael adducts in high yields (85–98%) and moderate to good enantioselectivities (16–94% ee), accompanied by an unexpected solvent-dependent inversion of the enantioselectivity.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
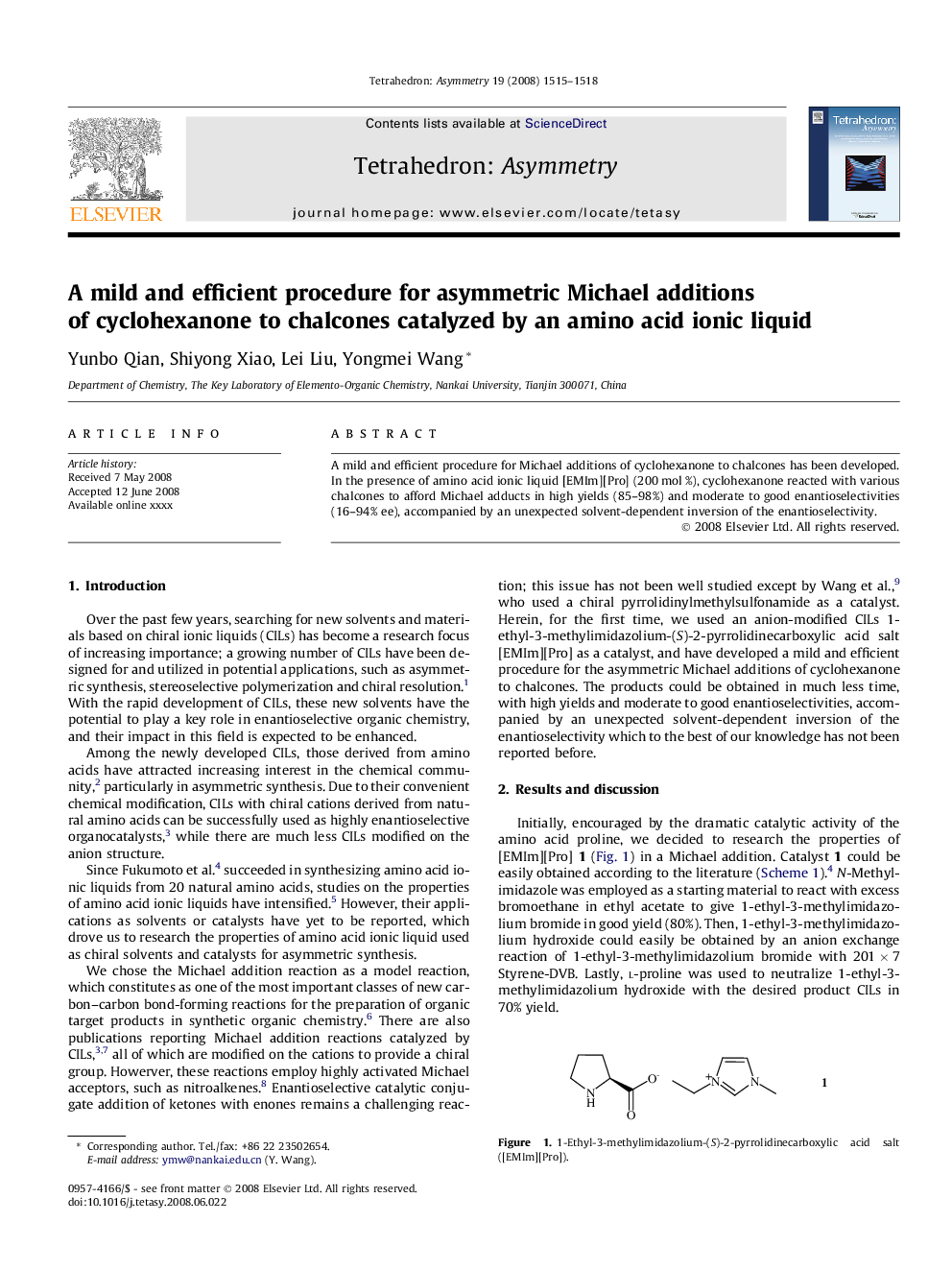
1-Ethyl-3-methylimidazolium-(S)-2-pyrrolidinecarboxylic acid saltC11H19N3O2[α]D25=+51.0 (c 1, MeOH)Source of chirality: l-proline
Related Topics
Physical Sciences and Engineering
Chemistry
Inorganic Chemistry
Authors
Yunbo Qian, Shiyong Xiao, Lei Liu, Yongmei Wang,