| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1347956 | Tetrahedron: Asymmetry | 2008 | 5 Pages |
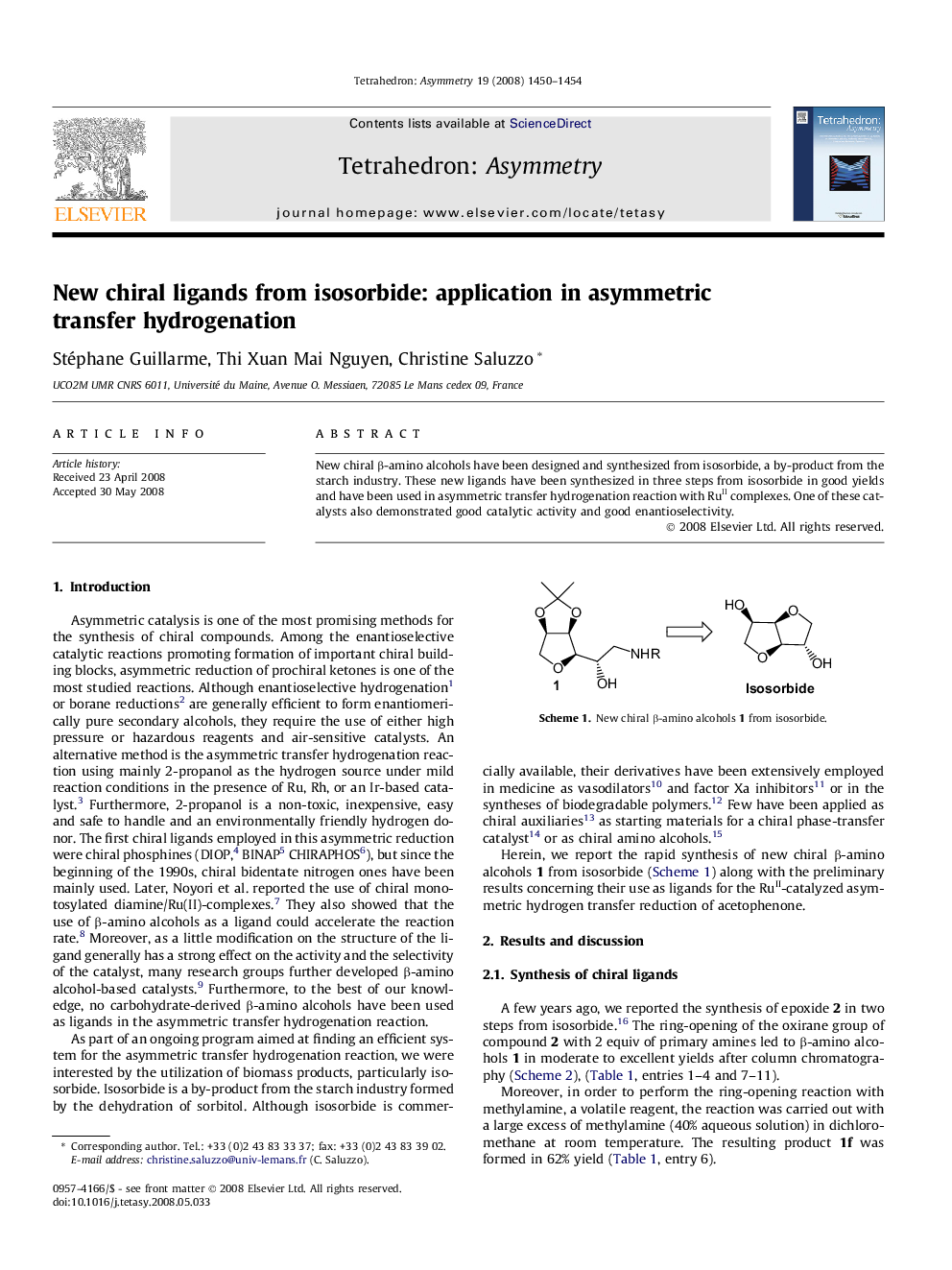
New chiral β-amino alcohols have been designed and synthesized from isosorbide, a by-product from the starch industry. These new ligands have been synthesized in three steps from isosorbide in good yields and have been used in asymmetric transfer hydrogenation reaction with RuII complexes. One of these catalysts also demonstrated good catalytic activity and good enantioselectivity.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
3,6-Anhydro-1-(benzylamino)-1-deoxy-4,5-O-isopropylidene-d-sorbitolC16H23NO4[α]D25=-57.5 (c 1.0, CH2Cl2)Source of chirality: isosorbideAbsolute configuration: (2S,3R,4R,5R)
3,6-Anhydro-1-[(S)-(1-hydroxybutan-2-ylamino)]-1-deoxy-4,5-O-isopropylidene-d-sorbitolC13H25NO5[α]D25=-35.3 (c 1.0, CH2Cl2)Source of chirality: isosorbideAbsolute configuration: (1′S,2S,3R,4R,5R)
3,6-Anhydro-1-(methylamino)-1-deoxy-4,5-O-isopropylidene-d-sorbitolC10H19NO4[α]D25=-54.2 (c 0.5, CH3OH)Source of chirality: isosorbideAbsolute configuration: (2S,3R,4R,5R)
3,6-Anhydro-1-(isopropylamino)-1-deoxy-4,5-O-isopropylidene-d-sorbitolC12H23NO4[α]D25=-58.8 (c 1.0, CH2Cl2)Source of chirality: isosorbideAbsolute configuration: (2S,3R,4R,5R)
3,6-Anhydro-1-(cyclohexylamino)-1-deoxy-4,5-O-isopropylidene-d-sorbitolC15H27NO4[α]D25=-58.2 (c 1.0, CH2Cl2)Source of chirality: isosorbideAbsolute configuration: (2S,3R,4R,5R)
3,6-Anhydro-1-[(R)-α-méthylbenzylamino)]-1-deoxy-4,5-O-isopropylidene-d-sorbitolC17H25NO4[α]D25=-17.3 (c 1.0, CH2Cl2)Source of chirality: isosorbideAbsolute configuration: (1′R,2S,3R,4R,5R)
3,6-Anhydro-1-(benzeneamino)-1-deoxy-4,5-O-isopropylidene-d-sorbitolC12H23NO4[α]D25=-70.0 (c 0.25, CH2Cl2)Source of chirality: isosorbideAbsolute configuration: (2S,3R,4R,5R)
3,6-Anhydro-1-(amino)-1-deoxy-4,5-O-isopropylidene-d-sorbitolC12H23NO4[α]D25=-40.0 (c 1.0, CH2Cl2)Source of chirality: isosorbideAbsolute configuration: (2S,3R,4R,5R)
3,6-Anhydro-1-(tertiobutylamino)-1-deoxy-4,5-O-isopropylidene-d-sorbitolC13H25NO4[α]D25=-62.3 (c 1.0, CH2Cl2)Source of chirality: isosorbideAbsolute configuration: (2S,3R,4R,5R)
3,6-Anhydro-1-((R)-(1-hydroxybutan-2-ylamino)-1-deoxy-4,5-O-isopropylidene-d-sorbitolC13H25NO5[α]D25=-64.0 (c 1.0, CH2Cl2)Source of chirality: isosorbideAbsolute configuration: (1′R,2S,3R,4R,5R)
3,6-Anhydro-1-(morpholino)-1-deoxy-4,5-O-isopropylidene-d-sorbitolC13H23NO5[α]D25=-63.8 (c 1.0, CH2Cl2)Source of chirality: isosorbideAbsolute configuration: (2S,3R,4R,5R)
3,6-Anhydro-1-[(S)-α-méthylbenzylamino)]-1-deoxy-4,5-O-isopropylidene-d-sorbitolC17H25NO4[α]D25=-90.0 (c 1.0, CH2Cl2)Source of chirality: isosorbideAbsolute configuration: (1′S,2S,3R,4R,5R)