| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1348161 | Tetrahedron: Asymmetry | 2006 | 8 Pages |
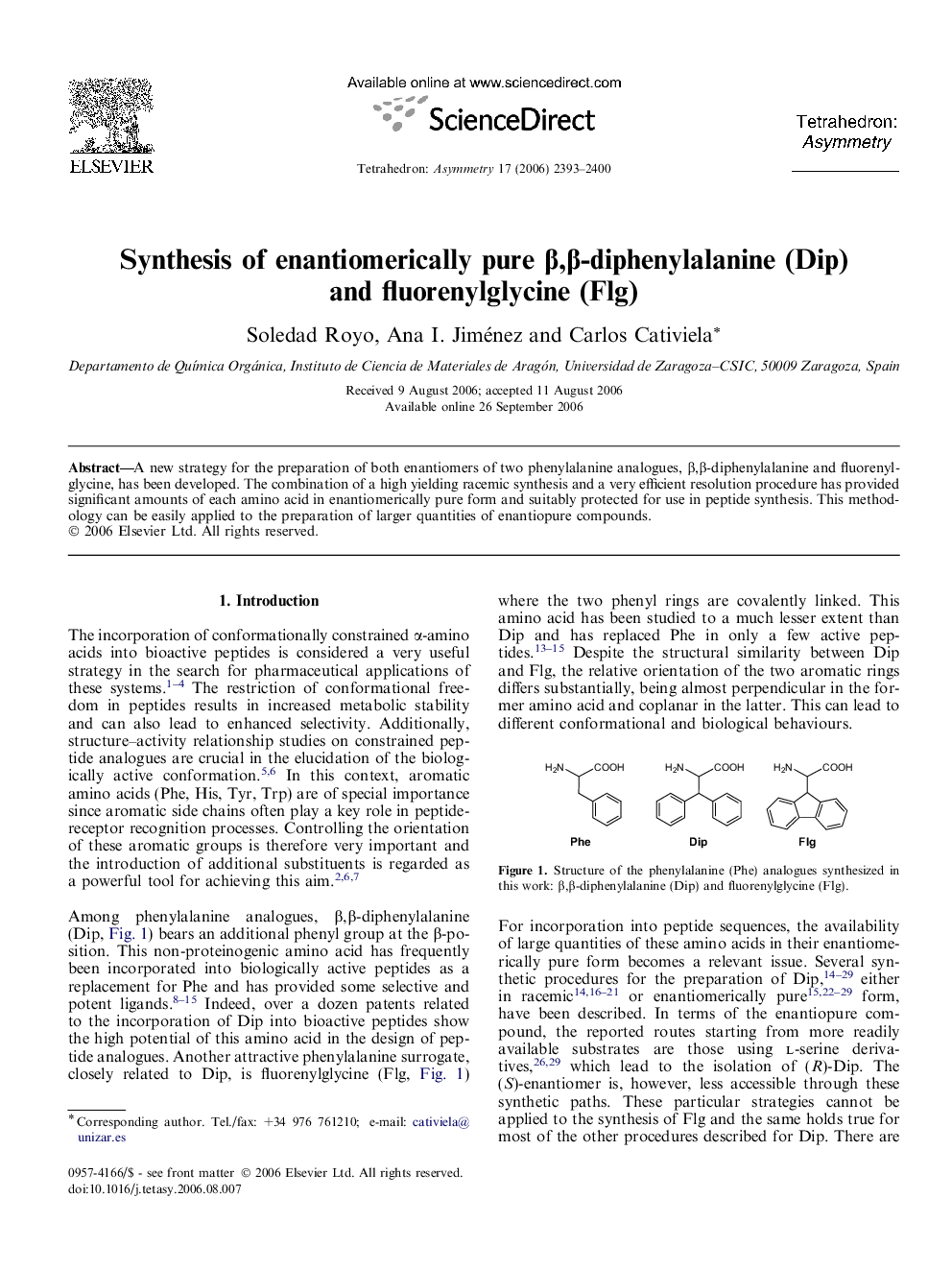
A new strategy for the preparation of both enantiomers of two phenylalanine analogues, β,β-diphenylalanine and fluorenylglycine, has been developed. The combination of a high yielding racemic synthesis and a very efficient resolution procedure has provided significant amounts of each amino acid in enantiomerically pure form and suitably protected for use in peptide synthesis. This methodology can be easily applied to the preparation of larger quantities of enantiopure compounds.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Benzyl (S)-2-(N-tert-butoxycarbonylamino)-3,3-diphenylpropanoateC27H29NO4Ee ⩾99%[α]D25 = +33.4 (c 0.62, CHCl3)Source of chirality: resolution by chiral HPLCAbsolute configuration: (S)
(S)-2-(N-tert-Butoxycarbonylamino)-3,3-diphenylpropanoic acidC20H23NO4Ee ⩾99%[α]D23 = +36.7 (c 0.59, MeOH)Source of chirality: resolution by chiral HPLCAbsolute configuration: (S)
Benzyl (S)-2-(N-tert-butoxycarbonylamino)-2-(9-fluorenyl)acetateC27H27NO4Ee ⩾99%[α]D25 = +3.4 (c 0.61, CHCl3)Source of chirality: resolution by chiral HPLCAbsolute configuration: (S)
(S)-2-(N-tert-Butoxycarbonylamino)-2-(9-fluorenyl)acetic acidC20H21NO4Ee ⩾99%[α]D23 = +53.9 (c 0.60, MeOH)Source of chirality: resolution by chiral HPLCAbsolute configuration: (S)