| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1348258 | Tetrahedron: Asymmetry | 2007 | 9 Pages |
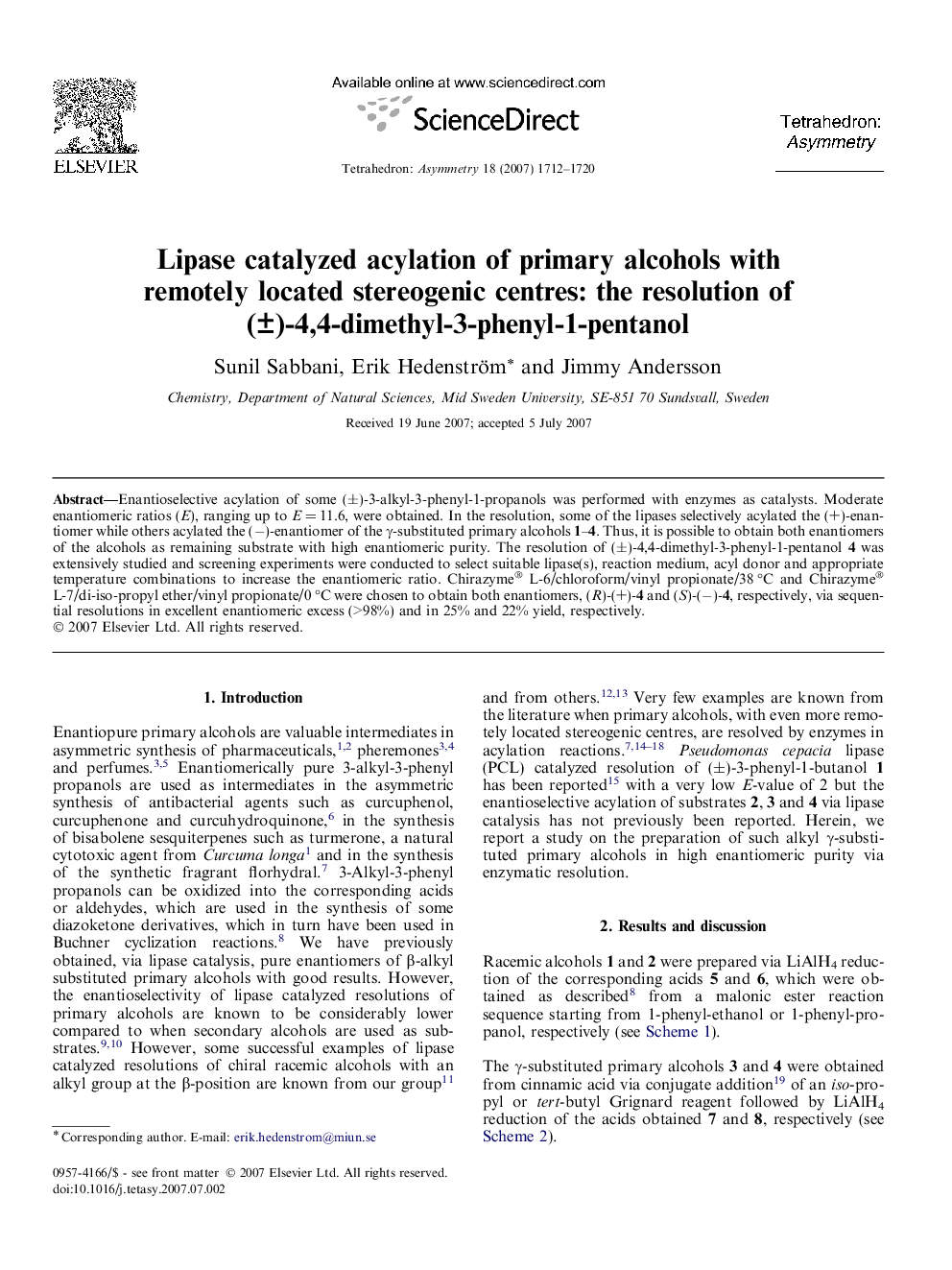
Enantioselective acylation of some (±)-3-alkyl-3-phenyl-1-propanols was performed with enzymes as catalysts. Moderate enantiomeric ratios (E), ranging up to E = 11.6, were obtained. In the resolution, some of the lipases selectively acylated the (+)-enantiomer while others acylated the (−)-enantiomer of the γ-substituted primary alcohols 1–4. Thus, it is possible to obtain both enantiomers of the alcohols as remaining substrate with high enantiomeric purity. The resolution of (±)-4,4-dimethyl-3-phenyl-1-pentanol 4 was extensively studied and screening experiments were conducted to select suitable lipase(s), reaction medium, acyl donor and appropriate temperature combinations to increase the enantiomeric ratio. Chirazyme® L-6/chloroform/vinyl propionate/38 °C and Chirazyme® L-7/di-iso-propyl ether/vinyl propionate/0 °C were chosen to obtain both enantiomers, (R)-(+)-4 and (S)-(−)-4, respectively, via sequential resolutions in excellent enantiomeric excess (>98%) and in 25% and 22% yield, respectively.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(S)-(+)-3-Phenyl-1-butanolC10H14O[α]D20=+11.5 (c 0.4, CDCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (S)
(S)-(+)-3-Phenyl-1-pentanolC11H16O[α]D20=+2.5 (c 0.8, CDCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (S)
(R)-(+)-4-Methyl-3-phenyl-1-pentanolC12H18O[α]D20=+7.25 (c 4.0, CDCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (R)
(R)-(+)-4,4-Dimethyl-3-phenyl-1-pentanolC13H20O[α]D20=+11.3 (c 4.0, benzene)Source of chirality: enzymatic resolutionAbsolute configuration: (R)