| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1348304 | Tetrahedron: Asymmetry | 2011 | 5 Pages |
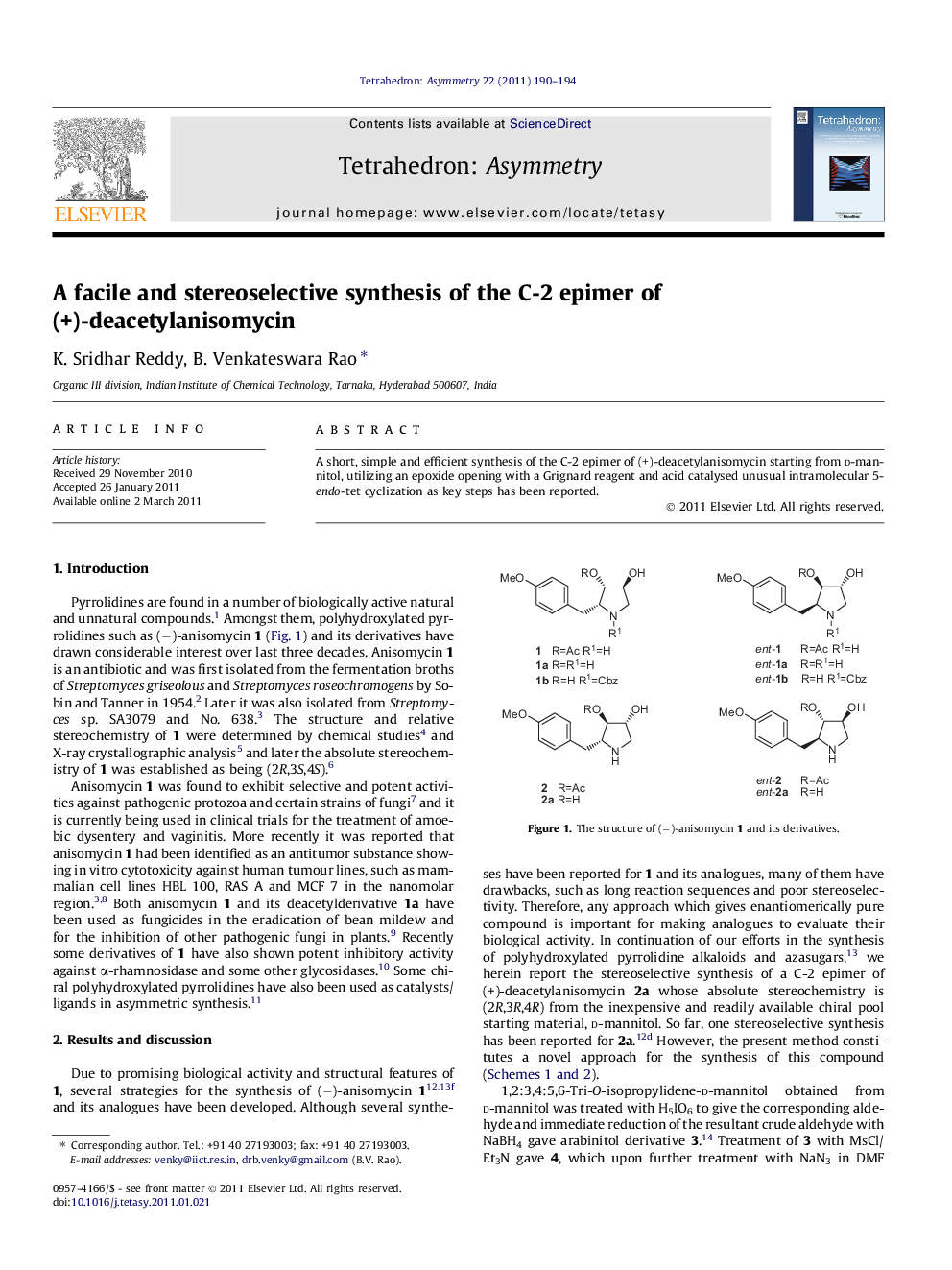
A short, simple and efficient synthesis of the C-2 epimer of (+)-deacetylanisomycin starting from d-mannitol, utilizing an epoxide opening with a Grignard reagent and acid catalysed unusual intramolecular 5-endo-tet cyclization as key steps has been reported.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
((4S,4′R,5R)-2,2,2′,2′-Tetramethyl-4,4′-bi(1,3-dioxolan)-5-yl)methanolC11H20O5[α]D25=-1.1 (c 1.0, CHCl3)Source of chirality: d-(+)-mannitolAbsolute configuration: (4S,4′R,5R)
(4S,4′R,5R)-5-(Azidomethyl)-2,2,2′,2′-tetramethyl-4,4′-bis(1,3-dioxolane)C11H19N3O4[α]D25=+63.4 (c 1.5, CHCl3)Source of chirality: d-(+)-mannitolAbsolute configuration: (4S,4′R,5R)
Benzyl ((4S,4′R,5R)-2,2,2′,2′-tetramethyl-4,4′-bi(1,3-dioxolan)-5-yl)methylcarbamateC19H27NO6[α]D25=-2.8 (c 1.8, CHCl3)Source of chirality: d-(+)-mannitolAbsolute configuration: (4S,4′R,5R)
Benzyl ((4R,5R)-5-((R)-1,2-dihydroxyethyl)-2,2-dimethyl-1,3-dioxolan-4-yl)methylcarbamateC16H26NO6[α]D25=-17.5 (c 1.0, CHCl3)Source of chirality: d-(+)-mannitolAbsolute configuration: (4R,5R,R)
(R)-2-((4R,5R)-5-((Benzyloxycarbonylamino) methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)-2-hydroxyethyl 4-methylbenzenesulfonateC23H29NO8S[α]D25=-32.3 (c 1.2, CHCl3)Source of chirality: d-(+)-mannitolAbsolute configuration: (R,4R,5R)
Benzyl ((4R,5S)-2,2-dimethyl-5-((R)-oxiran-2-yl)-1,3-dioxolan-4-yl) methylcarbamateC16H21NO5[α]D25=-4.5 (c 1.3, CHCl3)Source of chirality: d-(+)-mannitolAbsolute configuration: (4R,5S,R)
Benzyl ((4R,5R)-5-((R)-1-hydroxy-2-(4-methoxyphenyl)ethyl)-2,2-dimethyl-1,3-dioxolan-4-yl)methylcarbamateC23H29NO6[α]D25=-12.7 (c 1.0, CHCl3)Source of chirality: d-(+)-mannitolAbsolute configuration: (4R,5R,R)
(2R,3R,4R)-Benzyl 3,4-dihydroxy-2-(4-methoxybenzyl)pyrrolidine-1-carboxylateC20H23NO5[α]D25=+13.7 (c 1.1, CHCl3)Source of chirality: d-(+)-mannitolAbsolute configuration: (2R,3R,4R)
(2R,3R,4R)-2-(4-Methoxybenzyl)pyrrolidine-3,4-diolC12H17NO3[α]D25=+18.0 (c 0.8, CHCl3)Source of chirality: d-(+)-mannitolAbsolute configuration: (2R,3R,4R)
(2R,3R,4R)-Benzyl 3,4-dimethoxy-2-(4-methoxybenzyl)pyrrolidine-1-carboxylateC22H27NO5[α]D25=+30.2 (c 1.3, CHCl3)Source of chirality: d-(+)-mannitolAbsolute configuration: (2R,3R,4R)