| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1348336 | Tetrahedron: Asymmetry | 2007 | 10 Pages |
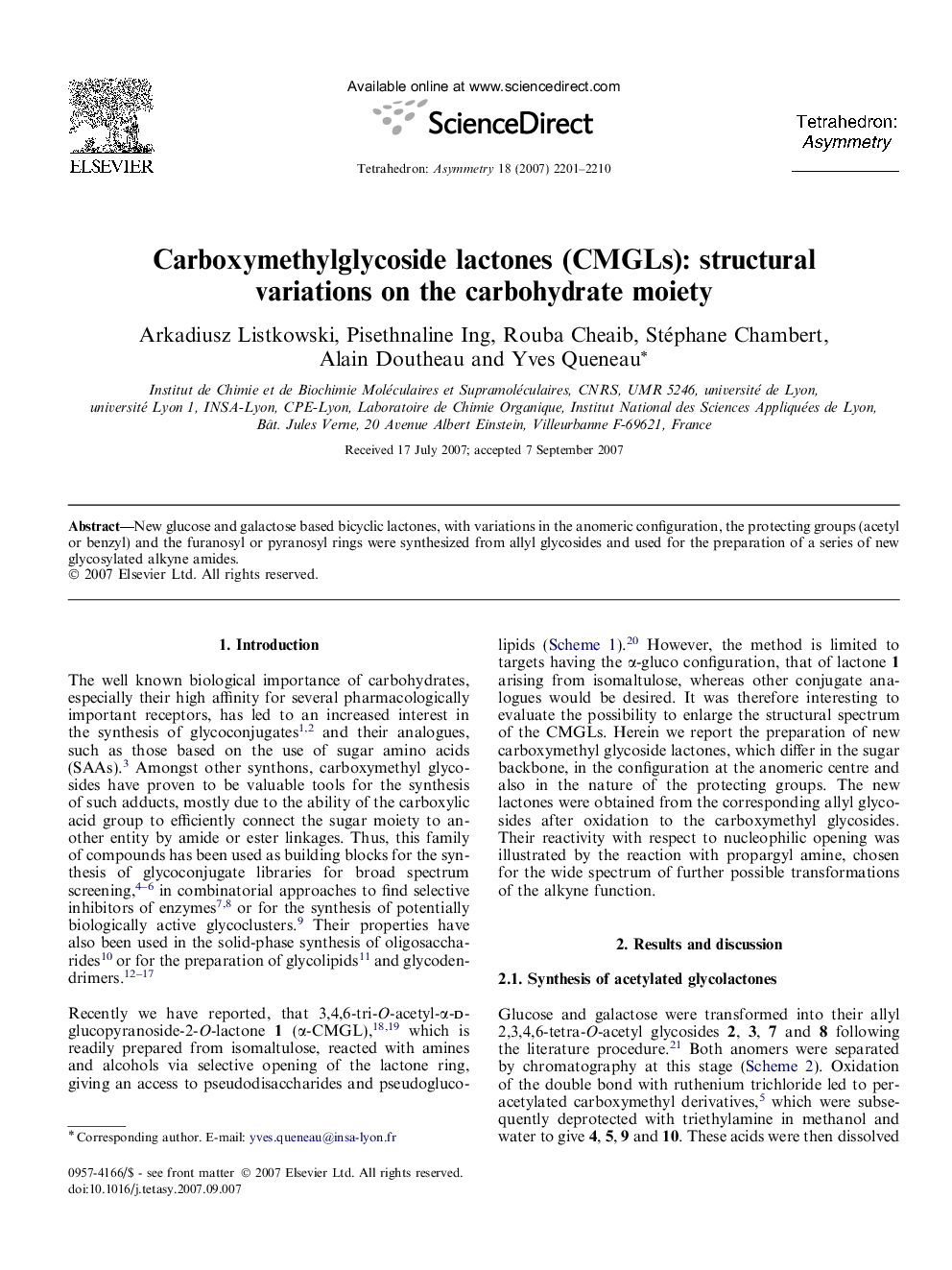
New glucose and galactose based bicyclic lactones, with variations in the anomeric configuration, the protecting groups (acetyl or benzyl) and the furanosyl or pyranosyl rings were synthesized from allyl glycosides and used for the preparation of a series of new glycosylated alkyne amides.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Carboxymethyl-3,4,6-tri-O-acetyl-α-d-galactopyranoside-2-O-lactoneC14H18O10Ee = 100%[α]D=+113[α]D=+113 (c 0.9, CHCl3)Source of chirality: allyl-α-d-galactopyranoside
Carboxymethyl-3,4,6-tri-O-acetyl-β-d-glucopyranoside-2-O-lactoneC14H18O10Ee = 100%[α]D=+93[α]D=+93 (c 1, CH2Cl2)Source of chirality: allyl-β-d-glucopyranoside
Carboxymethyl-3,4,6-tri-O-acetyl-β-d-galactopyranoside-2-O-lactoneC14H18O10Ee = 100%[α]D=+85[α]D=+85 (c 1, CHCl3)Source of chirality: allyl-β-d-galactopyranoside
Carboxymethyl-3,4,6-tri-O-benzyl-β-d-glucopyranoside-2-O-lactoneC29H30O7Ee = 100%[α]D=-21[α]D=-21 (c 1, CHCl3)Source of chirality: allyl-β-d-glucopyranoside
Carboxymethyl-3,4,6-tri-O-benzyl-β-d-galactopyranoside-2-O-lactoneC29H30O7Ee = 100%[α]D=+51[α]D=+51 (c 0.2, CHCl3)Source of chirality: allyl-β-d-galactopyranoside
Carboxymethyl-3,4,6-tri-O-benzyl-α-d-glucopyranoside-2-O-lactoneC29H30O7Ee = 100%[α]D=+88[α]D=+88 (c 0.8, CHCl3)Source of chirality: allyl-α-d-glucopyranoside
Carboxymethyl-3,4,6- tri-O-benzyl-α-d-galactopyranoside-2-O-lactoneC29H30O7Ee = 100%[α]D=+71[α]D=+71 (c 0.8, CHCl3)Source of chirality: allyl-α-d-galactopyranoside
Carboxymethyl-3,5,6-tri-O-benzyl-α-d-glucofuranoside-2-O-lactoneC29H30O7Ee = 100%[α]D=+19[α]D=+19 (c 0.7, CHCl3)Source of chirality: allyl-α-d-glucofuranoside
(N-Propargylcarbamoyl)methyl-3,4,6-tri-O-acetyl-α-d-galactopyranosideC17H23O10NEe = 100%[α]D=+122[α]D=+122 (c 0.6, CHCl3)Source of chirality: carboxymethyl-3,4,6-tri-O-acetyl-α-d-galactopyranoside-2-O-lactone
(N-Propargylcarbamoyl)methyl-3,4,6-tri-O-acetyl-β-d-galactopyranosideC17H23O10NEe = 100%[α]D=-2[α]D=-2 (c 0.6, CHCl3)Source of chirality: carboxymethyl-3,4,6-tri-O-acetyl-β-d-galactopyranoside-2-O-lactone
(N-Propargylcarbamoyl)methyl-3,4,6-tri-O-benzyl-β-d-glucopyranosideC32H35O7NEe = 100%[α]D=+5[α]D=+5 (c 0.3, CHCl3)Source of chirality: carboxymethyl-3,4,6-tri-O-benzyl-β-d-glucopyranoside-2-O-lactone
(N-Propargylcarbamoyl)methyl-3,4,6-tri-O-benzyl-β-d-galactopyranosideC32H35O7NEe = 100%[α]D=+3[α]D=+3 (c 1, CHCl3)Source of chirality: carboxymethyl-3,4,6-tri-O-benzyl-β-d-galactopyranoside-2-O-lactone
(N- Propargylcarbamoyl)methyl-3,4,6-tri-O-benzyl-α-d-glucopyranosideC32H35O7NEe = 100%[α]D=+88[α]D=+88 (c 0.5, CHCl3)Source of chirality: carboxymethyl-3,4,6-tri-O-benzyl-α-d-glucopyranoside-2-O-lactone
(N-Propargylcarbamoyl)methyl-3,4,6-tri-O-benzyl-α-d-galactopyranosideC32H35O7NEe = 100%[α]D=+84[α]D=+84 (c 0.6, CHCl3)Source of chirality: carboxymethyl-3,4,6-tri-O-benzyl-α-d-galactopyranoside-2-O-lactone
(N-Propargylcarbamoyl)methyl-3,4,6-tri-O-benzyl-α-d-glucofuranosideC32H35O7NEe = 100%[α]D=+10[α]D=+10 (c 0.9, CHCl3)Source of chirality: carboxymethyl-3,5,6-tri-O-benzyl-α-d-glucofuranoside-2-O-lactone
(N-Propargylcarbamoyl)methyl-α-d-glucopyranosideC11H17O7NEe = 100%[α]D=+188[α]D=+188 (c 1, H2O)Source of chirality: (N-propargylcarbamoyl)methyl-3,4,6-tri-O-acetyl-α-d-glucopyranoside
N-Methyl[-4-[1-(5′-deoxyuridin)-1,2,3-triazole]]carbamoylmethyl α-d-glucopyranosideC20H28O12N6Ee = 100%[α]D=+89[α]D=+89 (c 1, H2O)Source of chirality: (N-propargylcarbamoyl)methyl-α-d-glucopyranoside