| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1348643 | Tetrahedron: Asymmetry | 2014 | 9 Pages |
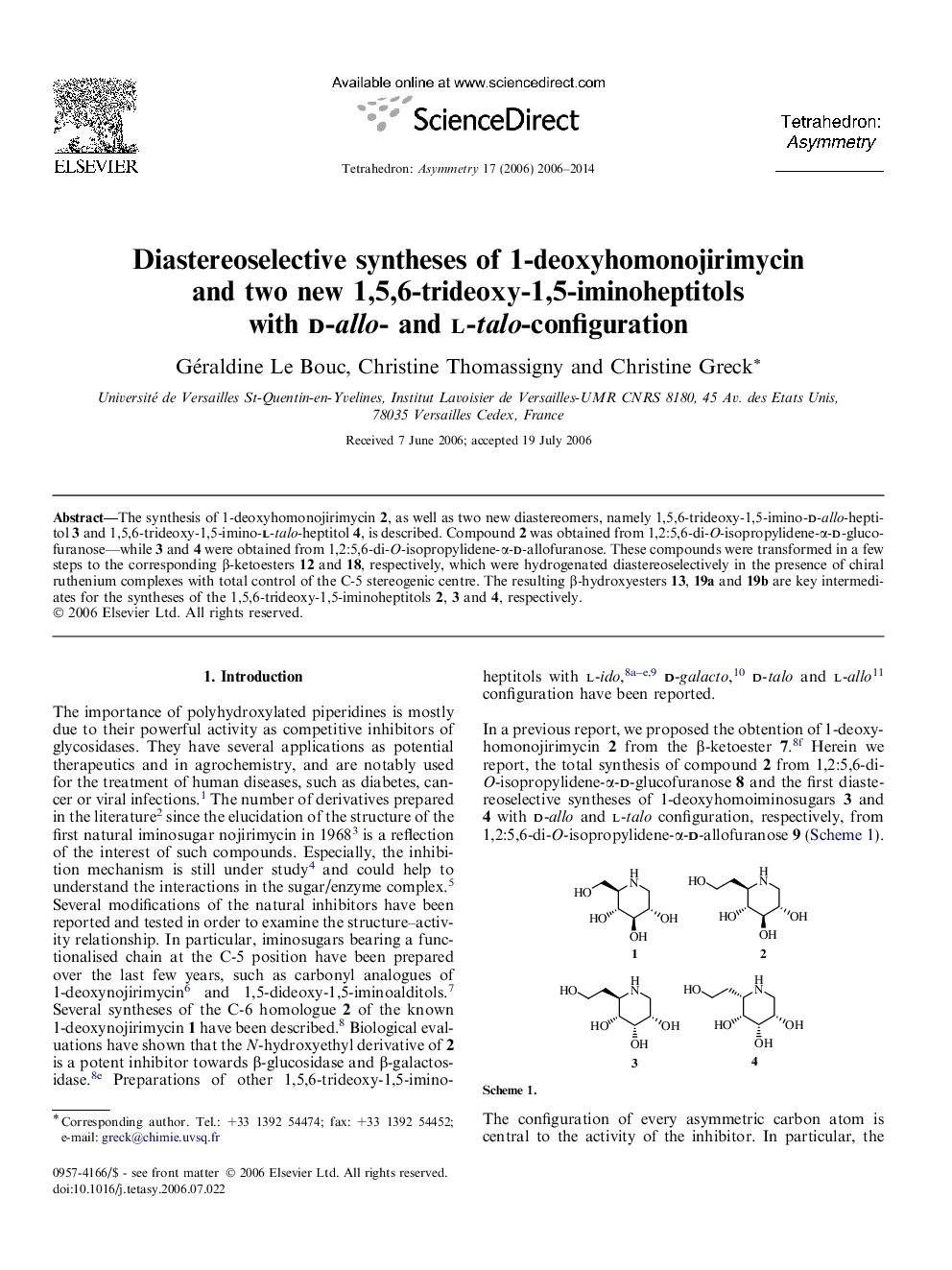
The synthesis of 1-deoxyhomonojirimycin 2, as well as two new diastereomers, namely 1,5,6-trideoxy-1,5-imino-d-allo-heptitol 3 and 1,5,6-trideoxy-1,5-imino-l-talo-heptitol 4, is described. Compound 2 was obtained from 1,2:5,6-di-O-isopropylidene-α-d-glucofuranose—while 3 and 4 were obtained from 1,2:5,6-di-O-isopropylidene-α-d-allofuranose. These compounds were transformed in a few steps to the corresponding β-ketoesters 12 and 18, respectively, which were hydrogenated diastereoselectively in the presence of chiral ruthenium complexes with total control of the C-5 stereogenic centre. The resulting β-hydroxyesters 13, 19a and 19b are key intermediates for the syntheses of the 1,5,6-trideoxy-1,5-iminoheptitols 2, 3 and 4, respectively.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
3-O-tert-Butyldimethylsilyl-1,2-O-isopropylidene-α-d-xylo-furanuronic acidC14H26O6Si[α]D = −48 (c 0.8, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-glucofuranoseAbsolute configuration: (1R,2R,3R,4S)
Methyl 3-O-tert-butyldimethylsilyl-6-deoxy-1,2-O-isopropylidene-5-oxo-α-d-xylo-heptofuranuronatesC17H30O7Si[α]D = −104 (c 1.4, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-glucofuranoseAbsolute configuration: (1R,2R,3R,4S)
Methyl 3-O-tert-butyldimethylsilyl-6-deoxy-1,2-O-isopropylidene-β-l-ido-heptofuranuronateC17H32O7SiDe >99%[α]D = −18 (c 1.6, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5S)
3-O-tert-Butyldimethylsilyl-6-deoxy-1,2-O-isopropylidene-β-l-ido-hepto-1,4-furanoseC16H32O6Si[α]D = −28 (c 1.1, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5S)
3-O-tert-Butyldimethylsilyl-7-O-tert-butyldiphenylsilyl-6-deoxy-1,2-O-isopropylidene-β-l-ido-hepto-1,4-furanoseC32H50O6Si[α]D = −18 (c 1.0, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5S)
5-Azido-3-O-tert-butyldimethylsilyl-7-O-tert-butyldiphenylsilyl-5,6-dideoxy-1,2-O-isopropylidene-α-d-gluco-hepto-1,4-furanoseC32H49N3O5Si2[α]D = −6 (c 1.6, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5R)
Methyl 3-O-benzyl-6-deoxy-1,2-O-isopropylidene-5-oxo-α-d-ribo-heptofuranuronateC18H22O7[α]D = +76 (c 1.3, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4S)
Methyl 3-O-benzyl-6-deoxy-1,2-O-isopropylidene-β-l-talo-heptofuranurinateC18H24O7De >99%[α]D = +72 (c 0.8, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-glucofuranoseAbsolute configuration: (1R,2R,3R,4R,5S)
Methyl 3-O-benzyl-6-deoxy-1,2-O-isopropylidene-α-d-allo-heptofuranuronateC18H24O7De >99%[α]D = +110 (c 0.6, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (1R,2R,3R,4R,5R)
3-O-Benzyl-6-deoxy-1,2-O-isopropylidene-β-l-talo-hepto-1,4-furanoseC17H24O6[α]D = +101 (c 0.9, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (1R,2R,3R,4R,5S)
3-O-Benzyl-6-deoxy-1,2-O-isopropylidene-α-d-allo-hepto-1,4-furanoseC17H24O6[α]D = +113 (c 1.0, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (1R,2R,3R,4R,5R)
3-O-Benzyl-6-deoxy-1,2-O-isopropylidene-7-O-triisopropylsilyl-β-l-talo-hepto-1,4-furanoseC26H44O6Si[α]D = +67 (c 1.0, DCM)Source of chirality: 1,2:5,6-di-O- isopropylidene-d-allofuranoseAbsolute configuration: (1R,2R,3R,4R,5S)
3-O-Benzyl-6-deoxy-1,2-O-isopropylidene-7-O-triisopropylsilyl-α-d-allo-hepto-1,4-furanoseC26H44O6Si[α]D = +64 (c 2.0, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (1R,2R,3R,4R,5R)
3-O-Benzyl-6-deoxy-1,2-O-isopropylidene-5-O-methanesulfonyl-7-O-triisopropylsilyl-β-l-talo-hepto-1,4-furanoseC27H46O8SSi[α]D = +41 (c 0.8, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (1R,2R,3R,4S,5S)
3-O-Benzyl-6-deoxy-1,2-O-isopropylidene-5-O-methanesulfonyl-7-O-triisopropylsilyl-α-d-allo-hepto-1,4-furanoseC27H46O8SSi[α]D = +61 (c 1.7, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (1R,2R,3R,4S,5R)
5-Azido-3-O-benzyl-5,6-dideoxy-1,2-O-isopropylidene-7-O-triisopropylsilyl-α-d-allo-hepto-1,4-furanoseC26H43N3O5Si[α]D = +105 (c 1.4, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (1R,2R,3R,4R,5R)
5-Azido-3-O-benzyl-5,6-dideoxy-1,2-O-isopropylidene-7-O-triisopropylsilyl-β-l-talo-hepto-1,4-furanoseC26H43N3O5Si[α]D = +48 (c 1.1, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (1R,2R,3R,4R,5S)
5-Azido-3-O-benzyl-5,6-dideoxy-1,2-O-isopropylidene-α-d-allo-hepto-1,4-furanoseC17H23N3O5[α]D = +152(c 2.1, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (1R,2R,3R,4R,5R)
5-Azido-3-O-benzyl-5,6-dideoxy-1,2-O-isopropylidene-β-l-talo-hepto-1,4-furanoseC17H23N3O5[α]D = +97 (c 2.0, DCM)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (1R,2R,3R,4R,5S)
1,5,6-Trideoxy-1,5-imino-d-allo-heptitol hydrochlorideC7H16CINO4[α]D = +19 (c 0.1, MeOH)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (2S,3S,4R,5R)
1,5,6-Trideoxy-1,5-imino-l-talo-heptitol hydrochlorideC7H16CINO4[α]D = +24 (c 0.6, MeOH)Source of chirality: 1,2:5,6-di-O-isopropylidene-d-allofuranoseAbsolute configuration: (2S,3S,4R,5S)