| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1348751 | Tetrahedron: Asymmetry | 2006 | 6 Pages |
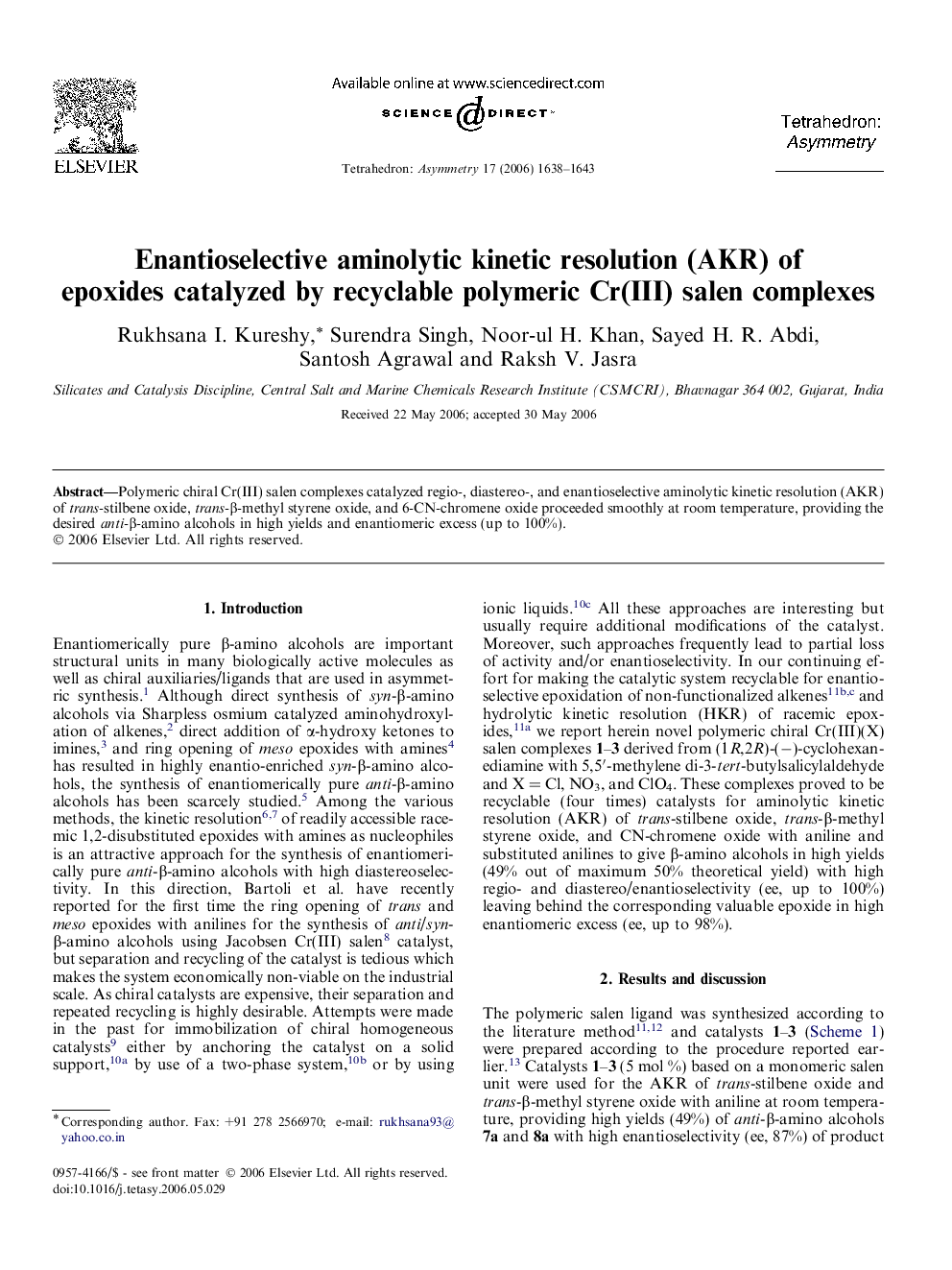
Polymeric chiral Cr(III) salen complexes catalyzed regio-, diastereo-, and enantioselective aminolytic kinetic resolution (AKR) of trans-stilbene oxide, trans-β-methyl styrene oxide, and 6-CN-chromene oxide proceeded smoothly at room temperature, providing the desired anti-β-amino alcohols in high yields and enantiomeric excess (up to 100%).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(1R,2R)-1,2-Diphenyl-oxiraneC14H12OEe = 98%Source of chirality: (1R,2R)-diaminocyclohexane[α]D27=+310 (c 2.5, C6H6)Absolute configuration: (1R,2R)
(1S,2R)-1,2-Diphenyl-2-phenylanilino-ethanolC20H19NOEe = 87%Source of chirality: (1R,2R)-diaminocyclohexane[α]D27=45.6 (c 1, CHCl3)Absolute configuration: (1S,2R)
(1S,2R)-1,2-Diphenyl-2-(2-methoxy-phenylanilino)-ethanolC21H21NO2Ee = 87%Source of chirality: (1R,2R)-diaminocyclohexane[α]D27=+26 (c 1.2, CHCl3)Absolute configuration: (1S,2R)
(1S,2R)-1,2-Diphenyl-2-(4-methoxy-phenylanilino)-ethanolC21H21NO2Ee = 87%Source of chirality: (1R,2R)-diaminocyclohexane[α]D27=+32.5 (c 1, CHCl3)Absolute configuration: (1S,2R)
(2R,3R)-2-Methyl-3-phenyl-oxiraneC9H10OEe = 92%Source of chirality: (1R,2R)-diaminocyclohexane[α]D27=+66 (c 1.0, CH2Cl2)Absolute configuration: (2R,3R)
(1R,2S)-1-Phenylanilino-1-phenyl-propan-2-olC15H17NOEe = 73%Source of chirality: (1R,2R)-diaminocyclohexane[α]D27=-20.2 (c 0.9, CHCl3)Absolute configuration: (1R,2S)
(1R,2S)-1-(2-Methoxy-phenylanilino)-1-phenyl-propan-2-olC16H19NO2Ee = 72%Source of chirality: (1R,2R)-diaminocyclohexane[α]D27=-19.5 (c 0.8, CHCl3)Absolute configuration: (1R,2S)
(1R,2S)-1-(4-Methoxy-phenylanilino)-1-phenyl-propan-2-olC16H19NO2Ee = 56%Source of chirality: (1R,2R)-diaminocyclohexane[α]D27=-15.8 (c 1.3, CHCl3)Absolute configuration: (1R,2S)
(3S,4S)-6-Cyano-2,2-dimethyl-3,4 epoxy-chromaneC12H11NO2Ee = 25%Source of chirality: (1R,2R)-diaminocyclohexane[α]D27=-6.9 (c 0.8, CH2Cl2)Absolute configuration: (3S,4S)
(3R,4S)-6-Cyano-2,2-dimethyl-3-(phenylanilino)-chromane-4-olC18H18N2O2Ee = 21%Source of chirality: (1R,2R)-diaminocyclohexane[α]D27=+14.6 (c 1.2, CH2Cl2)Absolute configuration: (3R,4S)