| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1349125 | Tetrahedron: Asymmetry | 2007 | 6 Pages |
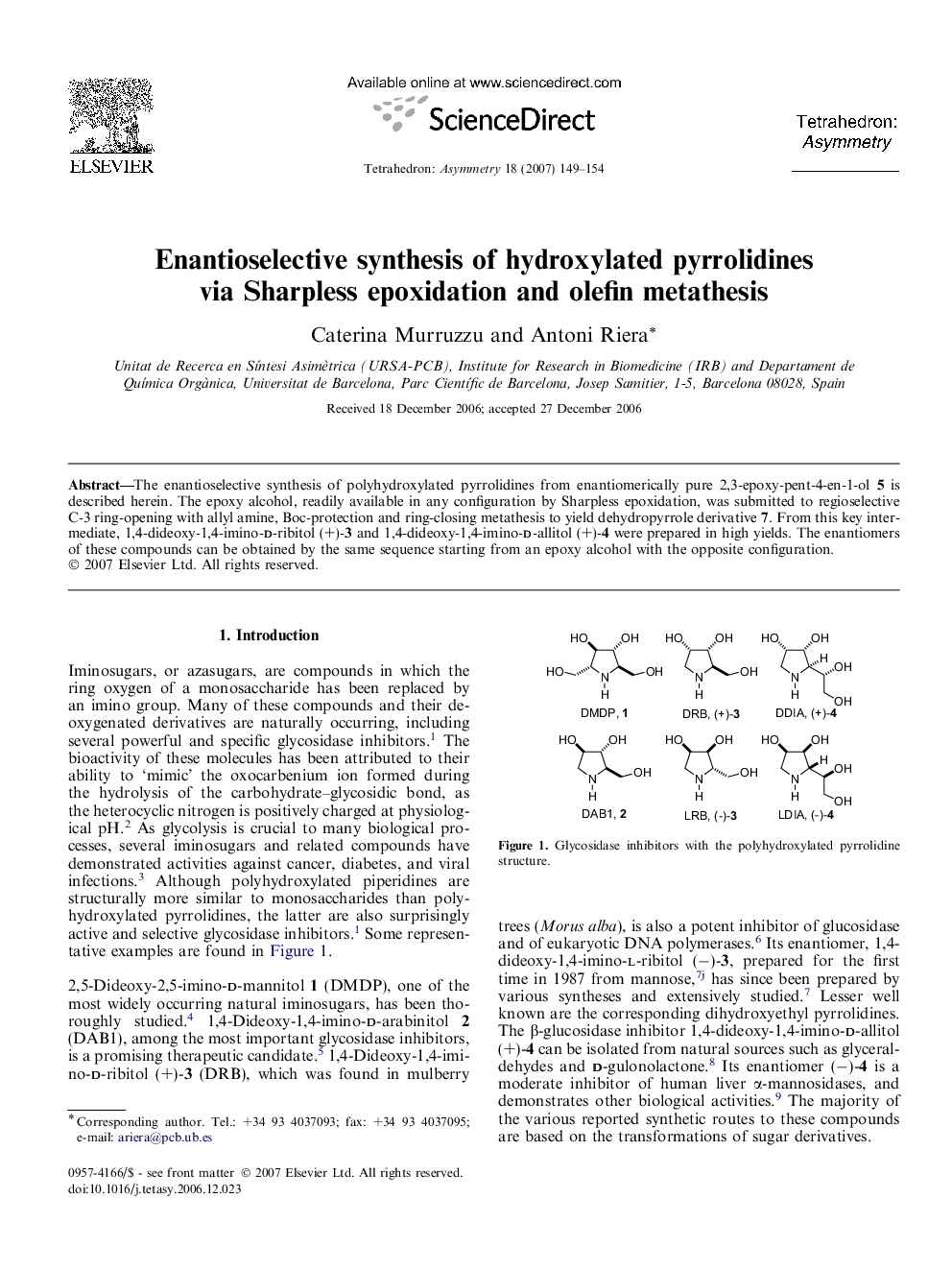
The enantioselective synthesis of polyhydroxylated pyrrolidines from enantiomerically pure 2,3-epoxy-pent-4-en-1-ol 5 is described herein. The epoxy alcohol, readily available in any configuration by Sharpless epoxidation, was submitted to regioselective C-3 ring-opening with allyl amine, Boc-protection and ring-closing metathesis to yield dehydropyrrole derivative 7. From this key intermediate, 1,4-dideoxy-1,4-imino-d-ribitol (+)-3 and 1,4-dideoxy-1,4-imino-d-allitol (+)-4 were prepared in high yields. The enantiomers of these compounds can be obtained by the same sequence starting from an epoxy alcohol with the opposite configuration.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(2S,3S)-3-(N-Allyl-N-tert-butoxycarbonyl)-4-penten-1,2-diolC13H23NO4[α]D = −22.6 (c 1.0, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2S,3S)
(2S)-N-tert-Butoxycarbonyl-2-[(1′S)-1′,2′-dihydroxyethyl]-2,5-dihydropyrroleC11H19NO4[α]D = −108.1 (c 0.97, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2S,1′S)
1,4-Dideoxy-1,4-imino-d-allitol bis-isopropylidene acetalC17H29NO6[α]D = −58.5 (c 1.0, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2R,3R,4S,1’S)
(2S)-N-tert-Butoxycarbonyl-2-hydroxymethyl-2,5-dihydro-1H-pyrroleC10H17NO3[α]D = −124.6 (c 1.0, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2S)
(2R,3R,4S)-N-tert-Butoxycarbonyl-2-hydroxymethyl-3,4-isopropylidendioxy-pyrrolidineC13H23NO3[α]D = −30.3 (c 0.3, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2R,3R,4S)
(2R)-N-tert-Butoxycarbonyl-2-tert-butyldiphenylsilyloxymethyl-2,5-dihydro-1H- pyrroleC26H36NO3Si[α]D = −24.6 (c 1.0, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2R)
(2R,3R,4S)-N-tert-Butoxycarbonyl-2-tert-butyldiphenylsilyloxymethyl-3,4-dihydroxypyrrolidine isopropyilidene acetalC29H41NO5Si[α]D = −36.1 (c 1.05, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2R,3R,4S)