| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1349259 | Tetrahedron: Asymmetry | 2010 | 6 Pages |
Chiral non-racemic 1,4-dihydropyridazines were prepared by the reaction of 1,2-diaza-1,3-dienes with arylacetaldehydes under organocatalytic conditions. l-Proline and (S)-(+)-1-(2-pyrrolidinylmethyl)pyrrolidine coupled with trifluoroacetic acid were used as organocatalysts. Enantiomeric excesses ranged from 25% to 78%.
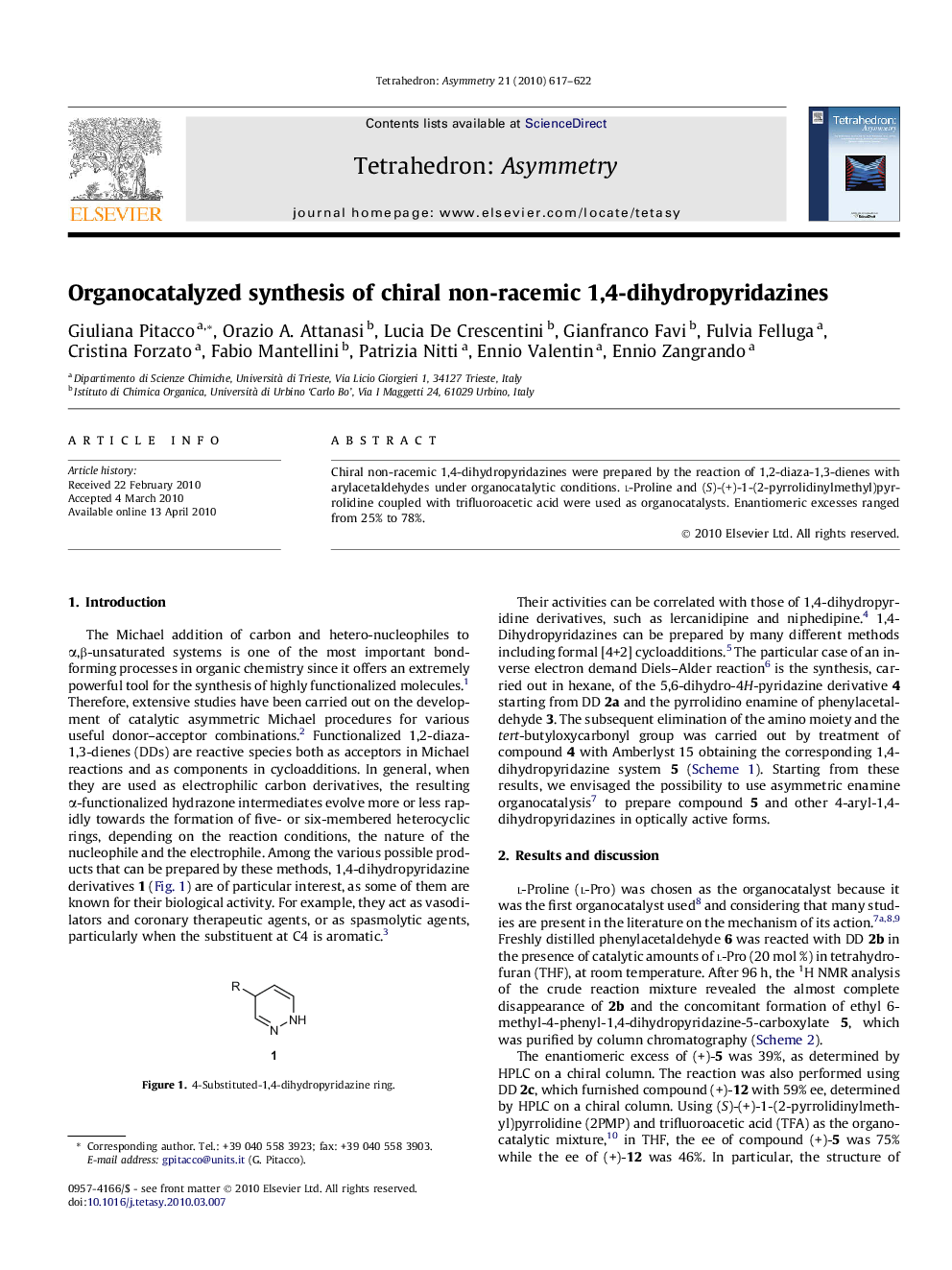
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Ethyl 6-methyl-4-phenyl-1,4-dihydropyridazine-5-carboxylateC14H16N2O2Ee = 75%[α]D20=+548.8 (c 0.25, MeOH)Source of chirality: organocatalysisAbsolute configuration: (S)
Methyl 6-methyl-4-phenyl-1,4-dihydropyridazine-5-carboxylateC13H14N2O2Ee = 46%[α]D20=+211.2 (c 0.34, MeOH)Source of chirality: organocatalysisAbsolute configuration: (S)
Methyl 4-(4-bromophenyl)-6-methyl-1,4-dihydropyridazine-5-carboxylateC13H13BrN2O2Ee = 72%[α]D20=+403.3 (c 0.45, MeOH)Source of chirality: organocatalysisAbsolute configuration: (S)
Methyl 4-(4-ethoxyphenyl)-6-methyl-1,4-dihydropyridazine-5-carboxylateC15H18N2O3Ee = 66%[α]D20=+198.0 (c 0.295, MeOH)Source of chirality: organocatalysisAbsolute configuration: (S)
Methyl 6-methyl-4-(4-nitrophenyl)-1,4-dihydropyridazine-5-carboxylateC13H13N3O4Ee = 69%[α]D20=+492.7 (c 0.055, MeOH)Source of chirality: organocatalysisAbsolute configuration: (S)
Methyl 6-methyl-4-(2-nitrophenyl)-1,4-dihydropyridazine-5-carboxylateC13H13N3O4Ee = 52%[α]D20=+71.5 (c 0.595, MeOH)Source of chirality: organocatalysisAbsolute configuration: (S)
Methyl 6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridazine-5-carboxylateC13H13N3O4Ee = 78%[α]D20=+419.9 (c 0.18, MeOH)Source of chirality: organocatalysisAbsolute configuration: (S)