| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1349889 | Tetrahedron: Asymmetry | 2005 | 4 Pages |
The enantioselective synthesis of 7-butyl-6,8-dihydroxy-(3R)-pentylisochroman-1-one has been achieved for the first time in which Sharpless asymmetric dihydroxylation is the key step.
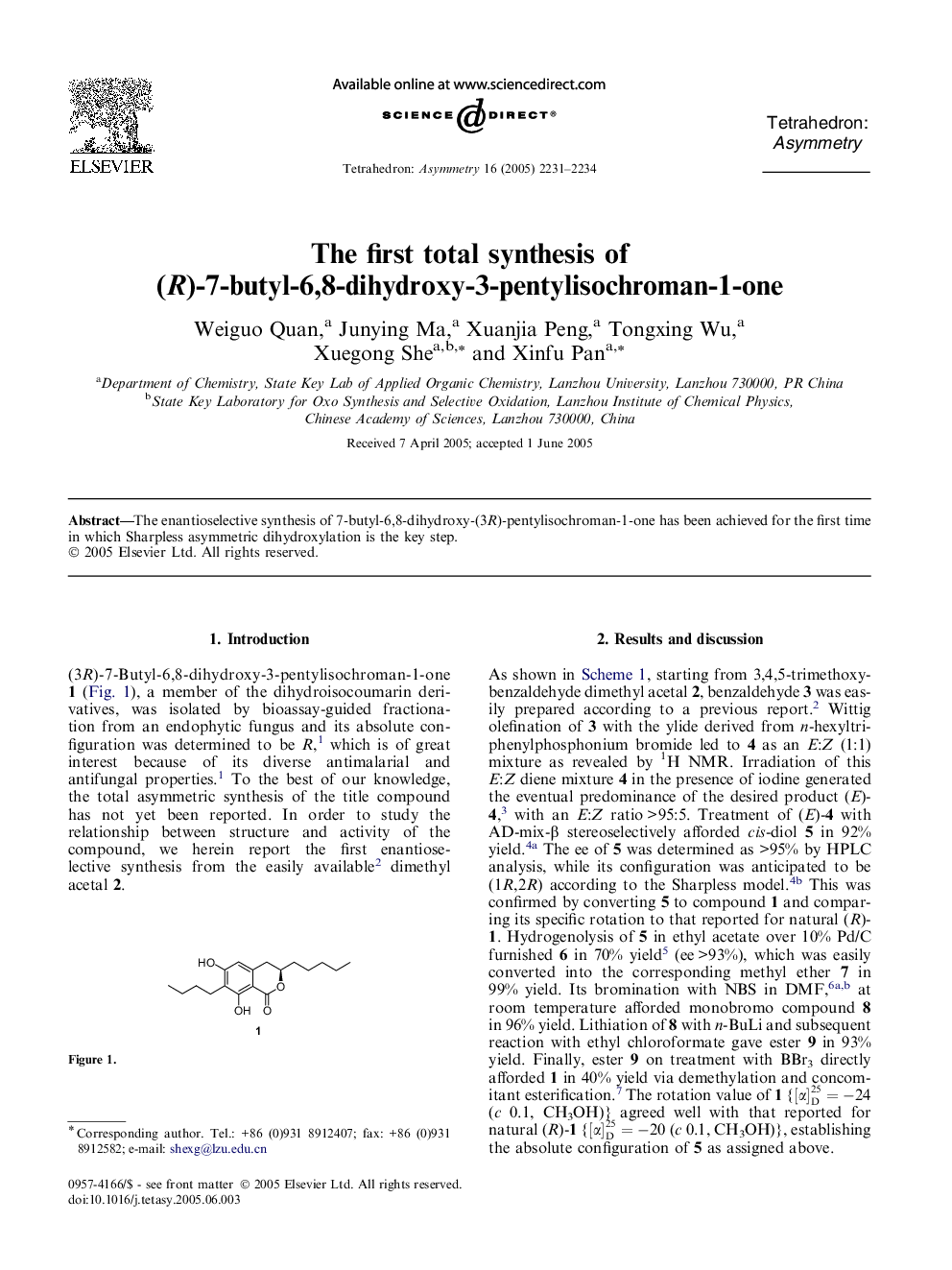
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(1R,2R)-1-(4-Butyl-3,5-dimethoxyphenyl)heptane-1,2-diolC19H32O4Ee 95%[α]D25=-3 (c 1.0, CH3OH)Source of chirality: enantioselective synthesisAbsolute configuration: 1R,2R
(R)-1-(4-Butyl-3,5-dimethoxyphenyl)heptan-2-olC19H32O3Ee 93%[α]D25=-12 (c 1.0, CH3OH)Source of chirality: enantioselective synthesisAbsolute configuration: R
2-Butyl-1,3-dimethoxy-5-((R)-2-methoxyheptyl)benzeneC20H34O3Ee 93%[α]D25=+14 (c 1.0, CH3OH)Source of chirality: enantioselective synthesisAbsolute configuration: R
2-Bromo-4-butyl-3,5-dimethoxy-1-((R)-2-methoxyheptyl)benzeneC20H33BrO3Ee 93%[α]D25=-4 (c 1.0, CH3OH)Source of chirality: enantioselective synthesisAbsolute configuration: R
Ethyl 3-butyl-2,4-dimethoxy-6-((R)-2-methoxyheptyl)benzoateC23H38O5Ee 93%[α]D25=+12 (c 1.02, CH3OH)Source of chirality: enantioselective synthesisAbsolute configuration: R
7-Butyl-6,8-dihydroxy-3(R)-pentylisochroman-1-oneC18H26O4Ee 93%[α]D25=-24 (c 0.1, CH3OH)Source of chirality: enantioselective synthesisAbsolute configuration: R