| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1349894 | Tetrahedron: Asymmetry | 2005 | 7 Pages |
Abstract
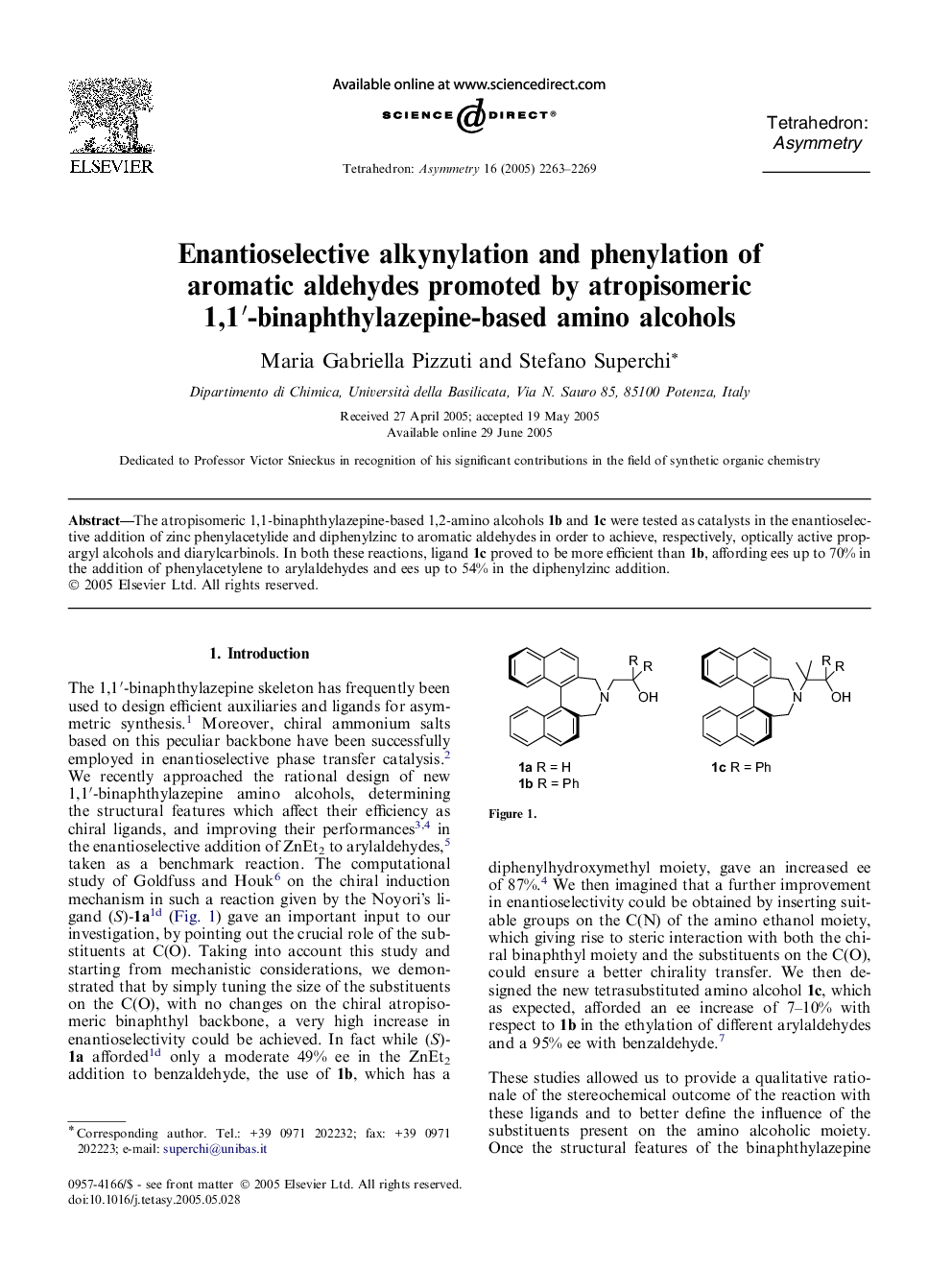
The atropisomeric 1,1-binaphthylazepine-based 1,2-amino alcohols 1b and 1c were tested as catalysts in the enantioselective addition of zinc phenylacetylide and diphenylzinc to aromatic aldehydes in order to achieve, respectively, optically active propargyl alcohols and diarylcarbinols. In both these reactions, ligand 1c proved to be more efficient than 1b, affording ees up to 70% in the addition of phenylacetylene to arylaldehydes and ees up to 54% in the diphenylzinc addition.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Inorganic Chemistry
Authors
Maria Gabriella Pizzuti, Stefano Superchi,