| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1350167 | Tetrahedron: Asymmetry | 2008 | 7 Pages |
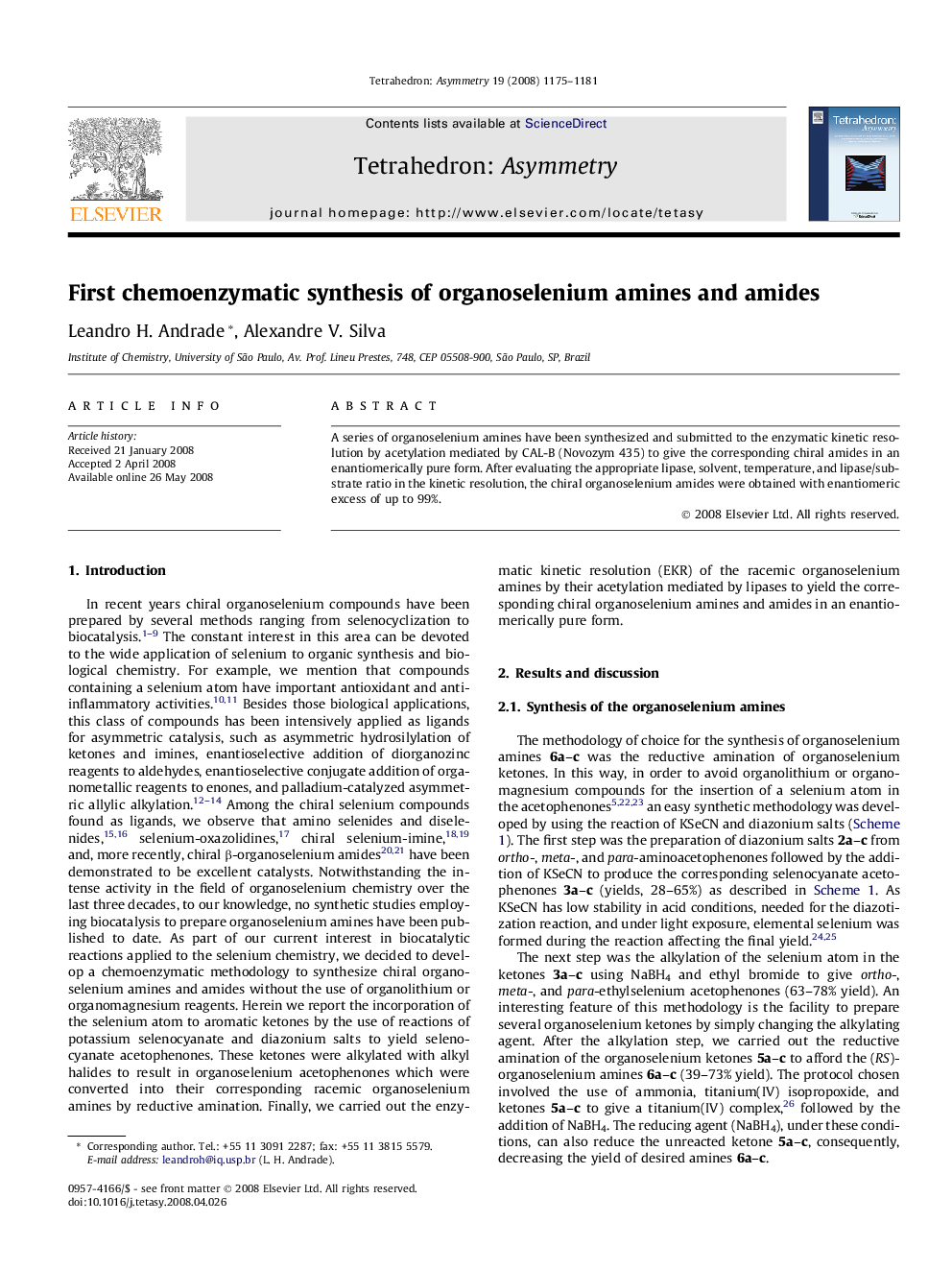
A series of organoselenium amines have been synthesized and submitted to the enzymatic kinetic resolution by acetylation mediated by CAL-B (Novozym 435) to give the corresponding chiral amides in an enantiomerically pure form. After evaluating the appropriate lipase, solvent, temperature, and lipase/substrate ratio in the kinetic resolution, the chiral organoselenium amides were obtained with enantiomeric excess of up to 99%.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(+)-(R)-N-(1-(4-(Ethylselanyl)phenyl)ethyl)acetamideC12H17NOSeEe = 99%[α]D24=+102.8 (c 0.59, ethyl acetate)Absolute configuration: (R)
(+)-(R)-N-(1-(3-(Ethylselanyl)phenyl)ethyl)acetamideC12H17NOSeEe = 98%[α]D24=+88.7 (c 0.21, ethyl acetate)Absolute configuration: (R)
(+)-(R)-N-(1-(2-(Ethylselanyl)phenyl)ethyl)acetamideC12H17NOSeEe = 99%[α]D24=+21.8 (c 0.5, ethyl acetate)Absolute configuration: (R)
(−)-(S)-1-(4-(Ethylselanyl)phenyl)ethanamineC10H15NSeEe = 22%[α]D26=-5.0 (c 0.5, ethyl acetate)Absolute configuration: (S)
(−)-(S)-1-(3-(Ethylselanyl)phenyl)ethanamineC10H15NSeEe = 34%[α]D26=-5.8 (c 2.24, ethyl acetate)Absolute configuration: (S)
(−)-(S)-1-(2-(Ethylselanyl)phenyl)ethanamineC10H15NSeEe = 08%[α]D26=-2.5 (c 3.49, ethyl acetate)Absolute configuration: (S)