| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1350175 | Tetrahedron: Asymmetry | 2008 | 9 Pages |
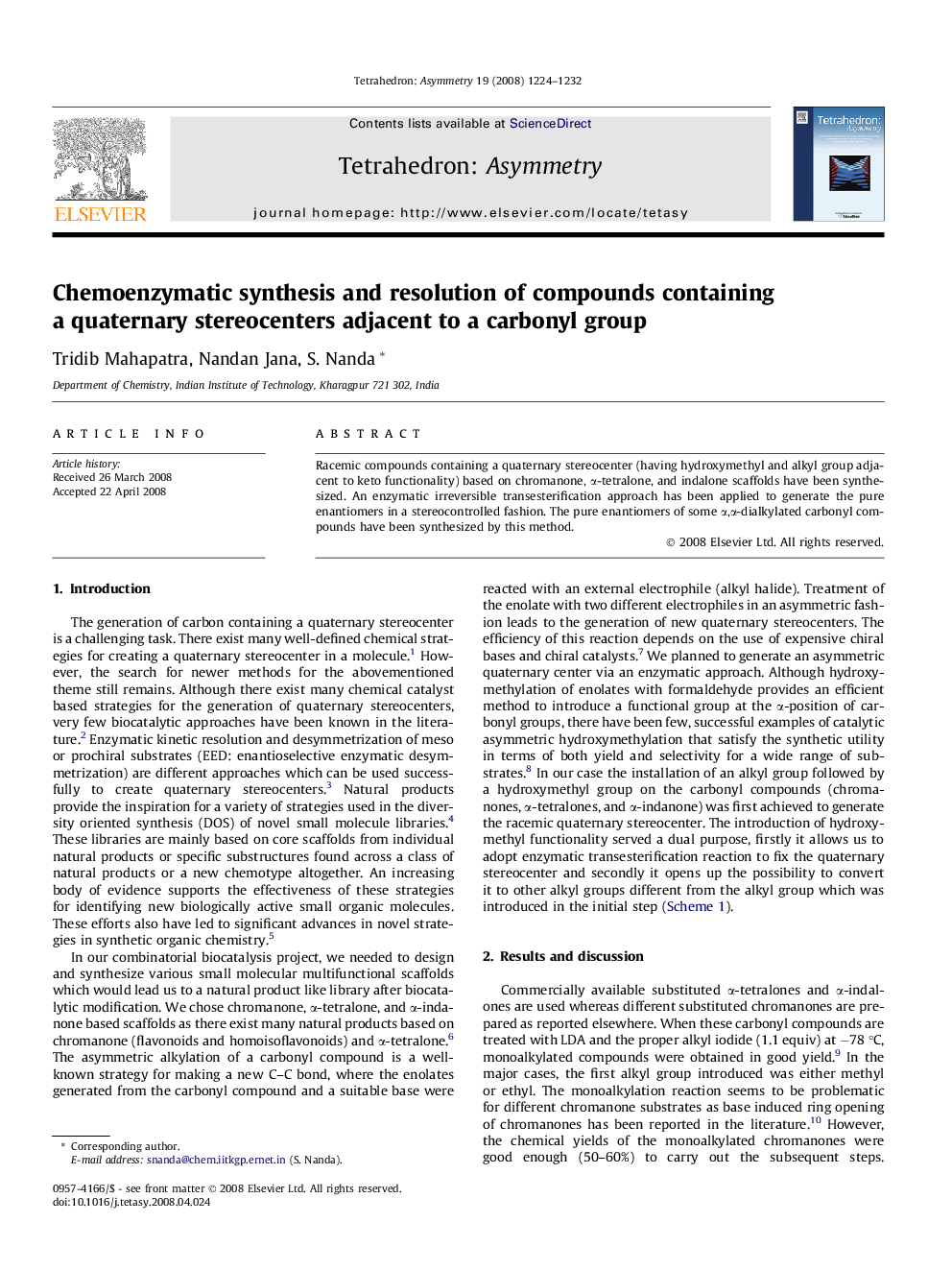
Racemic compounds containing a quaternary stereocenter (having hydroxymethyl and alkyl group adjacent to keto functionality) based on chromanone, α-tetralone, and indalone scaffolds have been synthesized. An enzymatic irreversible transesterification approach has been applied to generate the pure enantiomers in a stereocontrolled fashion. The pure enantiomers of some α,α-dialkylated carbonyl compounds have been synthesized by this method.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
(R)-2-Hydroxymethyl-2-methyl-3,4-dihydro-2H-naphthalen-1-oneC12H14O2Ee = 99%[α]D28=+1.8 (c 1.0, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (2R)
(R)-2-Hydroxymethyl-2-methyl-indan-1-oneC11H12O2Ee = 99%[α]D28=+4.1 (c 0.5, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (2R)
Acetic acid (R)-2-methyl-1-oxo-1,2,3,4-tetrahydro-naphthalen-2-ylmethyl esterC14H16O3Ee = 99%[α]D28=-11.1 (c 1.0, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (2R)
Acetic acid (R)-6-methoxy-2-ethyl-1-oxo-1,2,3,4-tetrahydro-naphthalen-2-ylmethyl esterC15H18O3Ee = 98%[α]D28=-18.5 (c 0.7, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (2R)
Acetic acid (R)-7-methoxy-2-methyl-1-oxo-1,2,3,4-tetrahydro-naphthalen-2-ylmethyl esterC15H18O3Ee = 98%[α]D28=-7.6 (c 0.9, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (2R)
Acetic acid (R)-5,8-dimethoxy-2-methyl-1-oxo-1,2,3,4-tetrahydro-naphthalen-2-ylmethyl esterC16H20O3Ee = 97%[α]D28=-2.3 (c 0.4, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (2R)
Acetic acid (R)-2-methyl-1-oxo-indan-2-ylmethyl esterC13H13O3Ee = 99%[α]D28=-5.6 (c 1.1, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (2R)
Acetic acid (R)-2-methyl-1-oxo-indan-2-ylmethyl esterC14H16O3Ee = 99%[α]D28=-18.8 (c 0.6, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (2R)
Acetic acid (R)-2-benzyl-5-chloro-1-oxo-indan-2-ylmethyl esterC19H17O3ClEe = 94%[α]D28=-12.6 (c 0.5, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (2R)
Acetic acid (R)-3-methyl-4-oxo-chroman-3-ylmethyl esterC13H14O4Ee = 98%[α]D28=-2.0 (c 0.5, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (3R)
Acetic acid (R)-7-chloro-3-methyl-4-oxo-chroman-3-ylmethyl esterC13H13O4ClEe = 95%[α]D28=-17.5 (c 1.0, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (3R)
Acetic acid (R)-7-methoxy-3-methyl-4-oxo-chroman-3-ylmethyl esterC14H16O5Ee = 98%[α]D28=-7.1 (c 0.5, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (3R)
Acetic acid (R)-6,7-dimethoxy-3-methyl-4-oxo-chroman-3-ylmethyl esterC15H18O6Ee = 98%[α]D28=-4.3 (c 0.6, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (3R)
Acetic acid (R)-7-methyl-8-oxo-7,8-dihydro-6H-[1,3]dioxolo[4,5-g]chromen-7-ylmethyl esterC14H14O6Ee = 96%[α]D28=-7.3 (c 0.8, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (3R)
(R)-2-Ethyl-6-methoxy-2-methyl-3,4-dihydro-2H-naphthalen-1-oneC14H18O2Ee = 98%[α]D28=-21.1 (c 1.2, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (2R)
(R)-2-Ethyl-2-methyl-indan-1-oneC12H14OEe = 99%[α]D28=-10.4 (c 1.0, MeOH)Source of chirality: enzymatic transesterificationAbsolute configuration: (2R)