| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1350272 | Tetrahedron: Asymmetry | 2008 | 5 Pages |
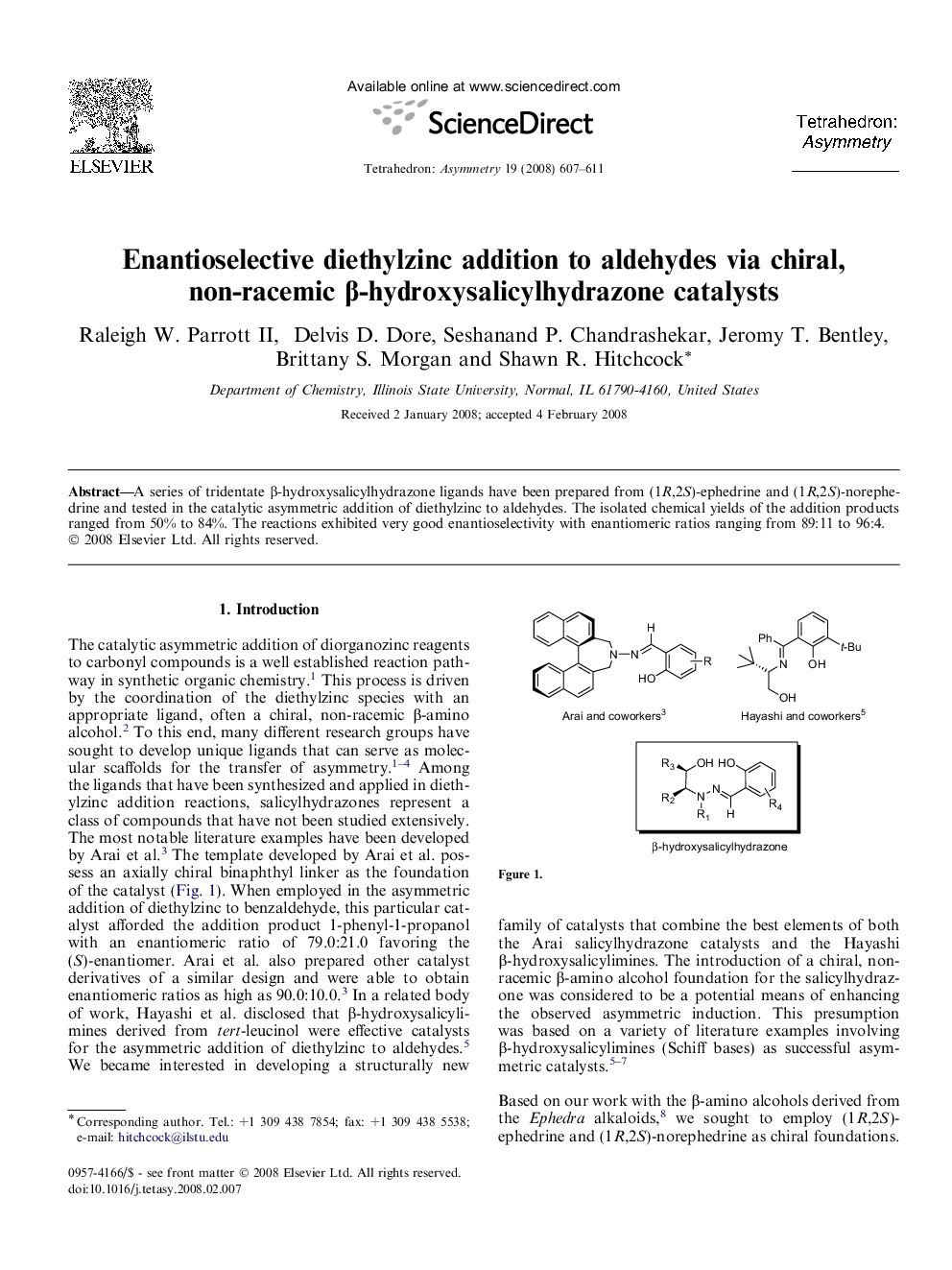
A series of tridentate β-hydroxysalicylhydrazone ligands have been prepared from (1R,2S)-ephedrine and (1R,2S)-norephedrine and tested in the catalytic asymmetric addition of diethylzinc to aldehydes. The isolated chemical yields of the addition products ranged from 50% to 84%. The reactions exhibited very good enantioselectivity with enantiomeric ratios ranging from 89:11 to 96:4.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
2-[(E)-(2-((1R,2S)-1-Hydroxy-1-phenyl-2-propyl)-2-methylhydrazono)methyl]phenolC17H20N2O2Yellow oil[α]D26=+233.6 (c 0.62, CHCl3)Source of chirality:(1R,2S)-ephedrineAbsolute Configuration:(1R,2S)
(1R,2S)-2-[(E)-2-Benzylidene-1-methylhydrazinyl]-1-phenylpropan-1-olC17H20N2OWhite solid, Mp = 51–53 °C[α]D26=+140.2 (c 0.71, CHCl3)Source of chirality:(1R,2S)-ephedrineAbsolute Configuration:(1R,2S)
2-[(E)-(2-((1R,2S)-1-Hydroxy-1-phenyl-2-propyl)-2-methylhydrazono)methyl]-6-methyl phenolC18H22N2O2Clear oil[α]D25=+219.7 (c 0.72, CHCl3)Source of chirality:(1R,2S)-ephedrineAbsolute Configuration:(1R,2S)
2-tert-Butyl-6-((E)-(2-((1R,2S)-1-hydroxy-1-phenyl-2-propyl)-2-methylhydrazono)methyl)phenolC21H28N2O2Yellow solid, Mp = 100–101 °C[α]D25=+191.9 (c 0.62, CHCl3)Source of chirality:(1R,2S)-ephedrineAbsolute Configuration:(1R,2S)
2,4-Di-tert-butyl-6-((E)-(2-((1R,2S)-1-hydroxy-1-phenyl-2-propyl)-2-methylhydrazono)methyl)phenolC25H36N2O2Clear oil[α]D25=+111.3 (c 0.80, CHCl3)Source of chirality:(1R,2S)-ephedrineAbsolute Configuration:(1R,2S)
2-((E)-(2-((1R,2S)-1-Hydroxy-1-phenyl-2-propyl)-2-methylhydrazono)methyl)-4-nitrophenolC25H35NO2Orange solid, Mp = 147–149 °C[α]D25=+180.4 (c 0.60, CHCl3)Source of chirality: (1R,2S)-ephedrineAbsolute Configuration: (1R,2S)
2-((E)-(2-((1R,2S)-1-Hydroxy-1-phenyl-2-propyl)-2-methylhydrazono)methyl)-4-methoxyphenolC18H22N2O3Clear oil[α]D25=+175.8 (c 0.59, CHCl3)Source of chirality: (1R,2S)-ephedrineAbsolute Configuration: (1R,2S)
1-((E)-(2-((1R,2S)-1-Hydroxy-1-phenyl-2-propyl)-2-methylhydrazono)methyl)naphthalen-2-olC21H22N2O2Dark yellow solid, Mp = 123–125 °C[α]D25=+192.8 (c 0.63, CHCl3)Source of chirality: (1R,2S)-ephedrineAbsolute Configuration: (1R,2S)
2-((E)-(2-((1R,2S)-1-Hydroxy-1-phenyl-2-propyl)-2-isopropylhydrazono)methyl)phenolC19H24N2O2Yellow oil[α]D25=+314.4 (c 0.70, CHCl3)Source of chirality: (1R,2S)-norephedrineAbsolute Configuration: (1R,2S)
1-((E)-(2-((1R,2S)-1-Hydroxy-1-phenyl-2-propyl)-2-isopropylhydrazono)methyl)naphthalene-2-olC23H26N2O2Yellow oil[α]D25=+196.6 (c 0.63, CHCl3)Source of chirality: (1R,2S)-norephedrineAbsolute Configuration: (1R,2S)