| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1350906 | Tetrahedron: Asymmetry | 2005 | 10 Pages |
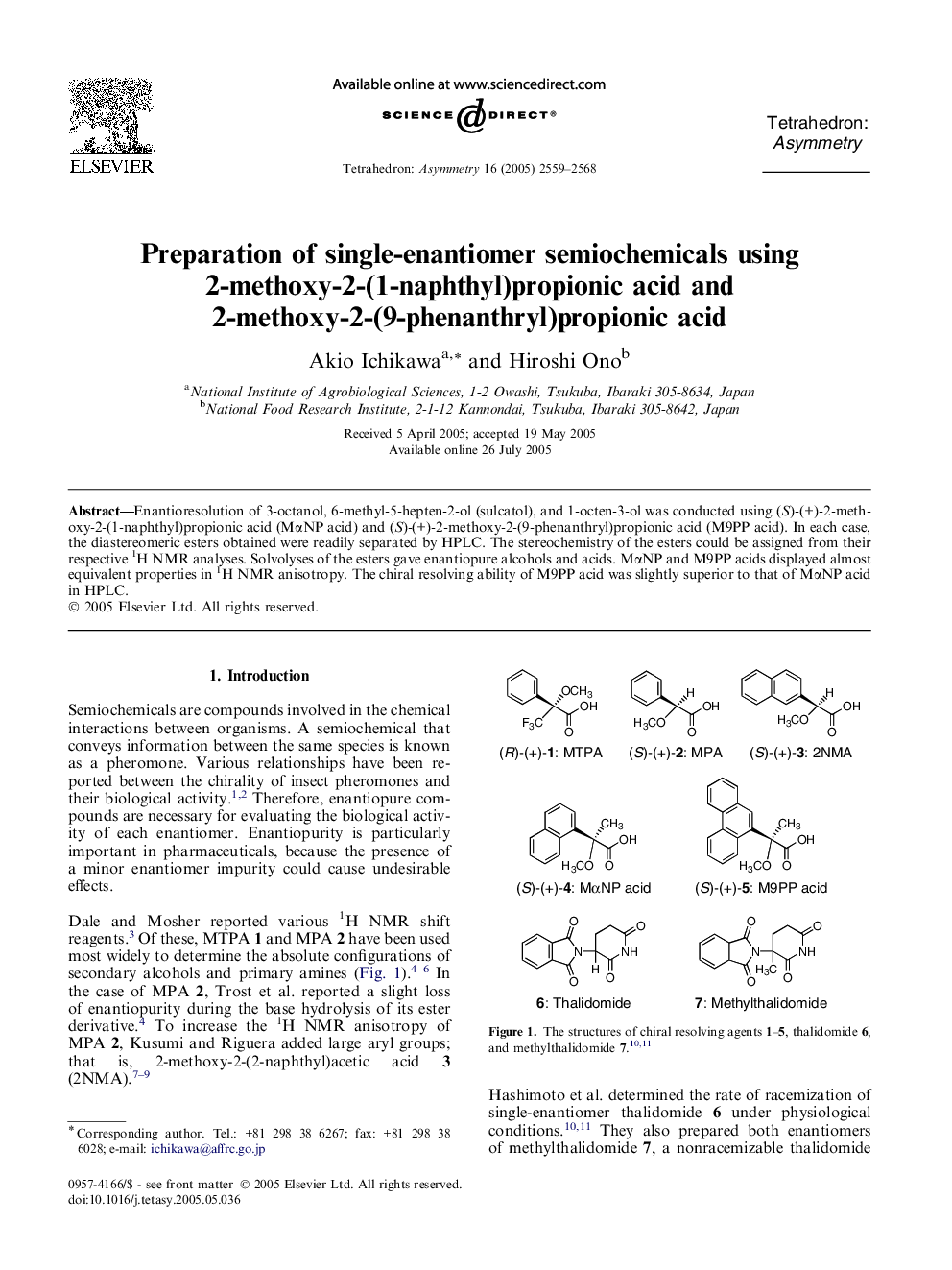
Enantioresolution of 3-octanol, 6-methyl-5-hepten-2-ol (sulcatol), and 1-octen-3-ol was conducted using (S)-(+)-2-methoxy-2-(1-naphthyl)propionic acid (MαNP acid) and (S)-(+)-2-methoxy-2-(9-phenanthryl)propionic acid (M9PP acid). In each case, the diastereomeric esters obtained were readily separated by HPLC. The stereochemistry of the esters could be assigned from their respective 1H NMR analyses. Solvolyses of the esters gave enantiopure alcohols and acids. MαNP and M9PP acids displayed almost equivalent properties in 1H NMR anisotropy. The chiral resolving ability of M9PP acid was slightly superior to that of MαNP acid in HPLC.
Graphical abstractEnantioresolution of the racemic alcohols was conducted using MαNP and M9PP acids. The stereochemistry of the esters was determind by the 1H NMR anisotropy method.Figure optionsDownload full-size imageDownload as PowerPoint slide
1-Ethylhexyl 2-methoxy-2-(1-naphthyl)propionateC22H30O3[α]D31-12 (c 0.68, EtOH)Source of chirality: synthesisAbsolute configuration: (2S,αR)
1-Ethylhexyl 2-methoxy-2-(1-naphthyl)propionateC22H30O3[α]D33+15 (c 0.72, EtOH)Source of chirality: synthesisAbsolute configuration: (2S,αS)
1,5-Dimethyl-4-hexenyl 2-methoxy-2-(1-naphthyl)propionateC22H28O3[α]D25-37 (c 0.54, EtOH)Source of chirality: synthesisAbsolute configuration: (2S,αR)
1,5-Dimethyl-4-hexenyl 2-methoxy-2-(1-naphthyl)propionateC22H28O3[α]D27+52 (c 0.46, EtOH)Source of chirality: synthesisAbsolute configuration: (2S,αS)
1-Vinylhexyl 2-methoxy-2-(9-phenanthryl)propionateC26H30O3[α]D28+32 (c 0.49, EtOH)Source of chirality: synthesisAbsolute configuration: (2S,αS)
1-Vinylhexyl 2-methoxy-2-(1-naphthyl)propionateC26H30O3[α]D28+34 (c 0.55, EtOH)Source of chirality: synthesisAbsolute configuration: (2S,αR)