| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1358603 | Bioorganic & Medicinal Chemistry | 2012 | 8 Pages |
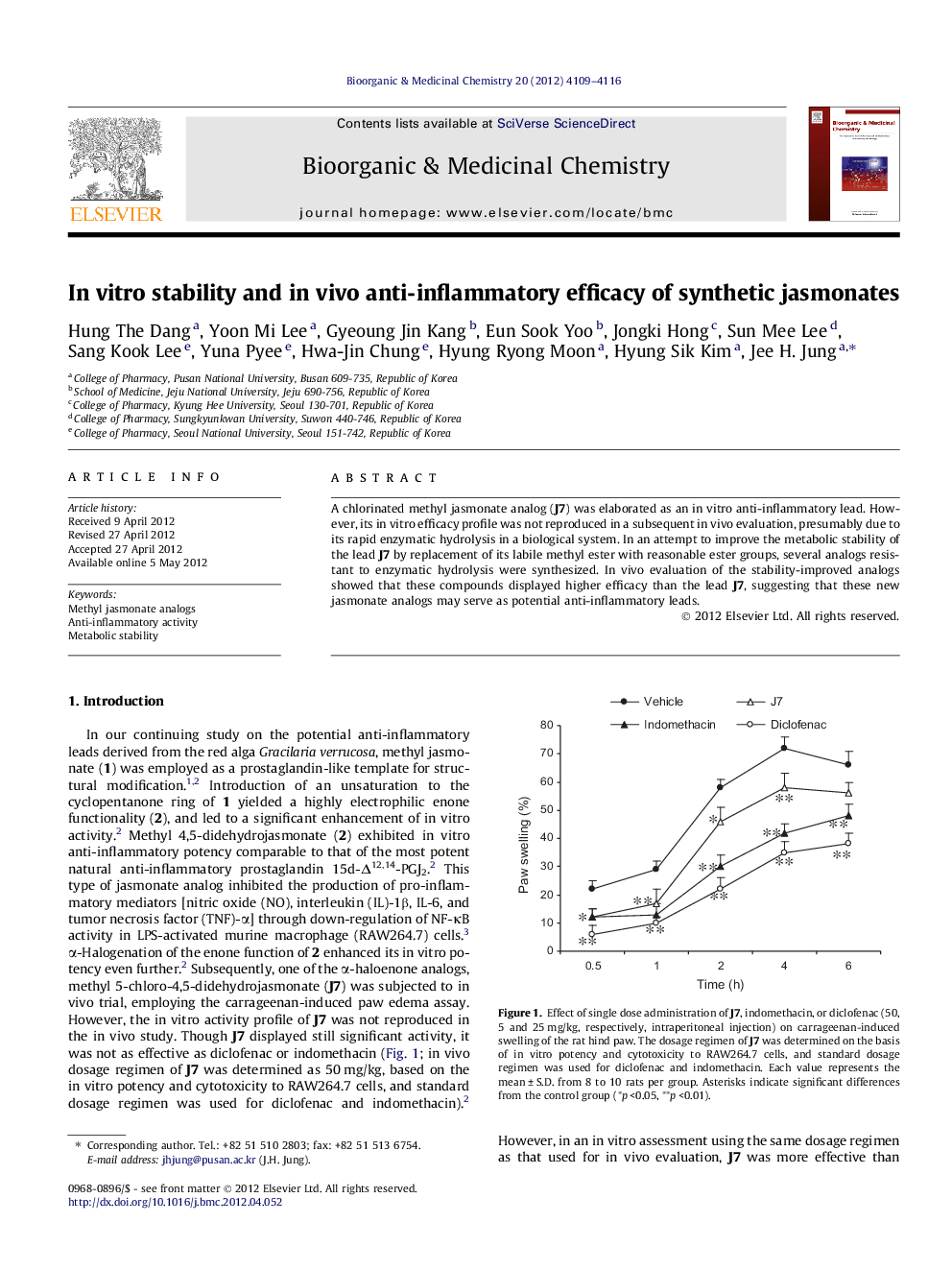
A chlorinated methyl jasmonate analog (J7) was elaborated as an in vitro anti-inflammatory lead. However, its in vitro efficacy profile was not reproduced in a subsequent in vivo evaluation, presumably due to its rapid enzymatic hydrolysis in a biological system. In an attempt to improve the metabolic stability of the lead J7 by replacement of its labile methyl ester with reasonable ester groups, several analogs resistant to enzymatic hydrolysis were synthesized. In vivo evaluation of the stability-improved analogs showed that these compounds displayed higher efficacy than the lead J7, suggesting that these new jasmonate analogs may serve as potential anti-inflammatory leads.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide