| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1386982 | Carbohydrate Research | 2017 | 6 Pages |
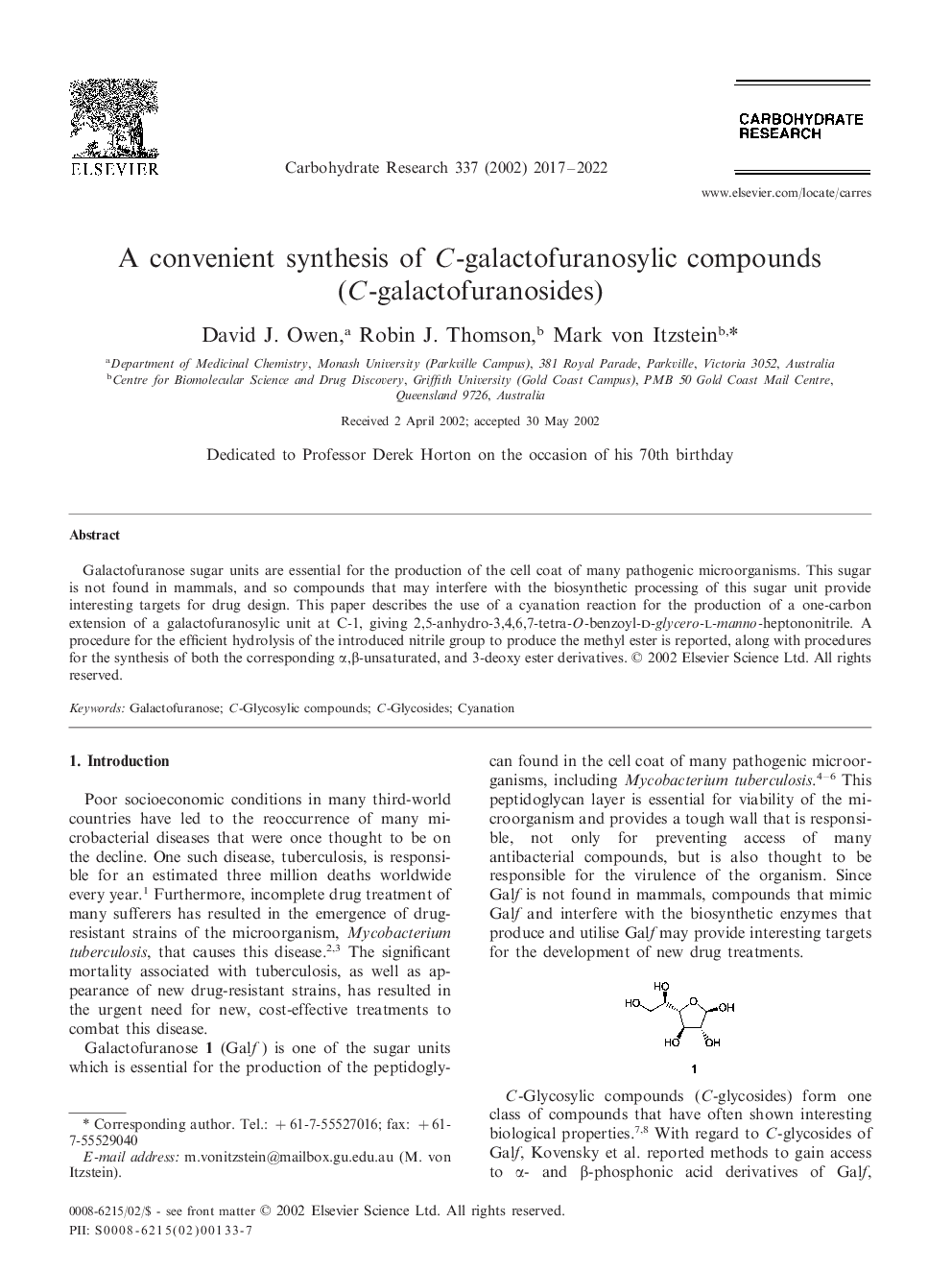
Galactofuranose sugar units are essential for the production of the cell coat of many pathogenic microorganisms. This sugar is not found in mammals, and so compounds that may interfere with the biosynthetic processing of this sugar unit provide interesting targets for drug design. This paper describes the use of a cyanation reaction for the production of a one-carbon extension of a galactofuranosylic unit at C-1, giving 2,5-anhydro-3,4,6,7-tetra-O-benzoyl-d-glycero-l-manno-heptononitrile. A procedure for the efficient hydrolysis of the introduced nitrile group to produce the methyl ester is reported, along with procedures for the synthesis of both the corresponding α,β-unsaturated, and 3-deoxy ester derivatives.
GraphicFigure optionsDownload full-size imageDownload as PowerPoint slide