| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1389511 | Carbohydrate Research | 2009 | 6 Pages |
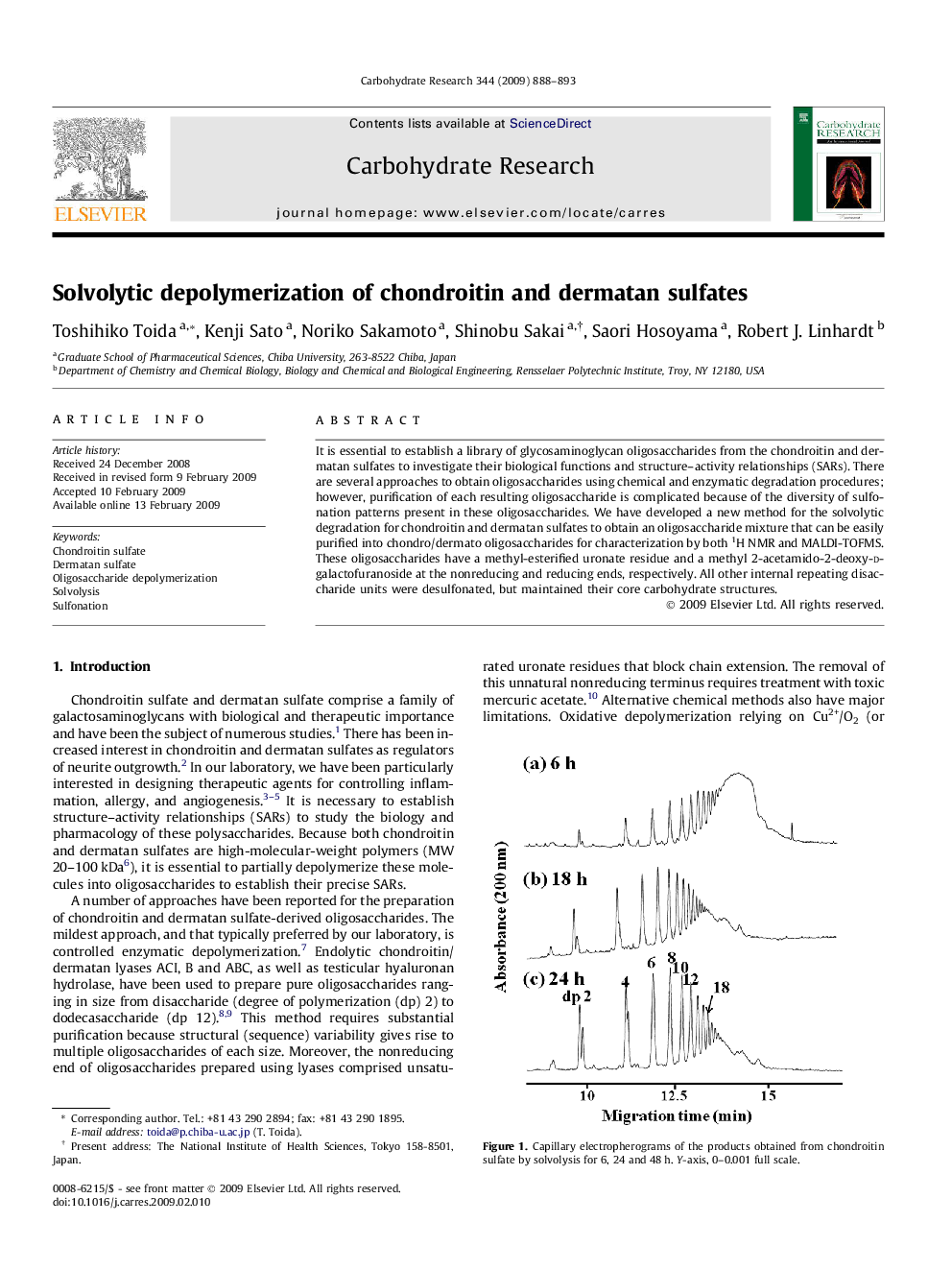
It is essential to establish a library of glycosaminoglycan oligosaccharides from the chondroitin and dermatan sulfates to investigate their biological functions and structure–activity relationships (SARs). There are several approaches to obtain oligosaccharides using chemical and enzymatic degradation procedures; however, purification of each resulting oligosaccharide is complicated because of the diversity of sulfonation patterns present in these oligosaccharides. We have developed a new method for the solvolytic degradation for chondroitin and dermatan sulfates to obtain an oligosaccharide mixture that can be easily purified into chondro/dermato oligosaccharides for characterization by both 1H NMR and MALDI-TOFMS. These oligosaccharides have a methyl-esterified uronate residue and a methyl 2-acetamido-2-deoxy-d-galactofuranoside at the nonreducing and reducing ends, respectively. All other internal repeating disaccharide units were desulfonated, but maintained their core carbohydrate structures.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide