| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1430152 | Materials Science and Engineering: C | 2009 | 4 Pages |
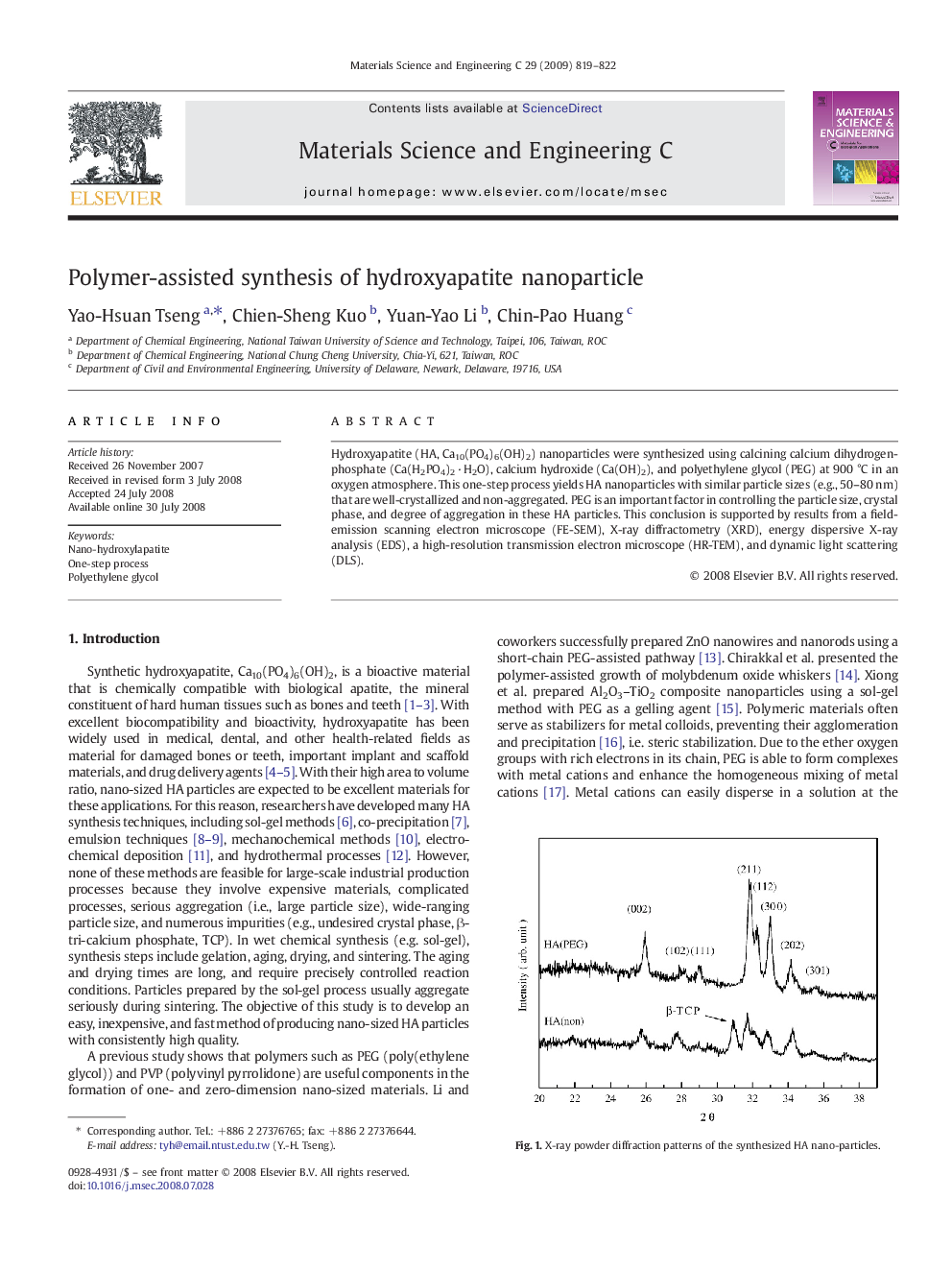
Hydroxyapatite (HA, Ca10(PO4)6(OH)2) nanoparticles were synthesized using calcining calcium dihydrogenphosphate (Ca(H2PO4)2 · H2O), calcium hydroxide (Ca(OH)2), and polyethylene glycol (PEG) at 900 °C in an oxygen atmosphere. This one-step process yields HA nanoparticles with similar particle sizes (e.g., 50–80 nm) that are well-crystallized and non-aggregated. PEG is an important factor in controlling the particle size, crystal phase, and degree of aggregation in these HA particles. This conclusion is supported by results from a field-emission scanning electron microscope (FE-SEM), X-ray diffractometry (XRD), energy dispersive X-ray analysis (EDS), a high-resolution transmission electron microscope (HR-TEM), and dynamic light scattering (DLS).