| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1470472 | Corrosion Science | 2009 | 8 Pages |
Abstract
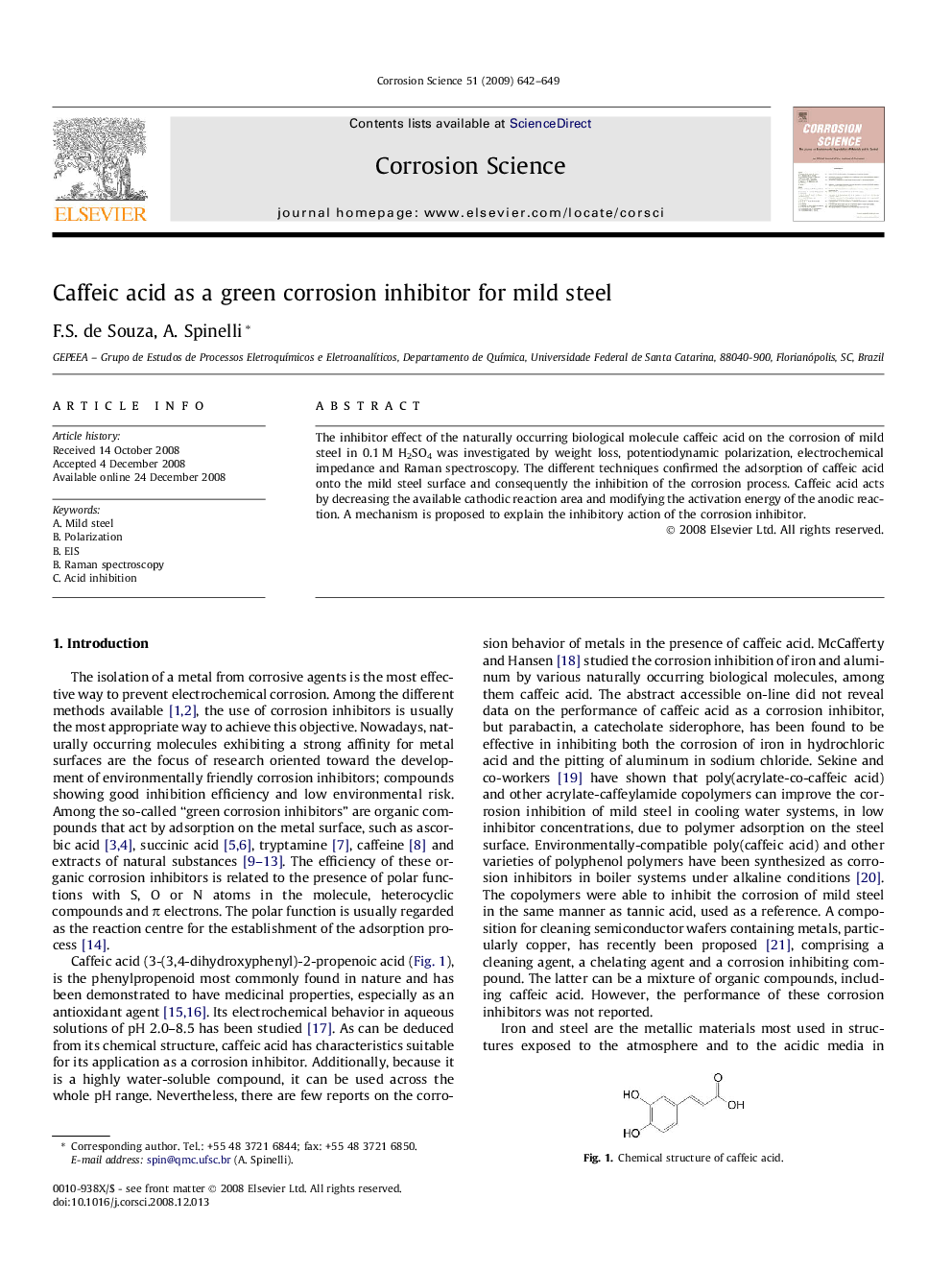
The inhibitor effect of the naturally occurring biological molecule caffeic acid on the corrosion of mild steel in 0.1 M H2SO4 was investigated by weight loss, potentiodynamic polarization, electrochemical impedance and Raman spectroscopy. The different techniques confirmed the adsorption of caffeic acid onto the mild steel surface and consequently the inhibition of the corrosion process. Caffeic acid acts by decreasing the available cathodic reaction area and modifying the activation energy of the anodic reaction. A mechanism is proposed to explain the inhibitory action of the corrosion inhibitor.
Related Topics
Physical Sciences and Engineering
Materials Science
Ceramics and Composites
Authors
F.S. de Souza, A. Spinelli,