| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1471640 | Corrosion Science | 2008 | 8 Pages |
Abstract
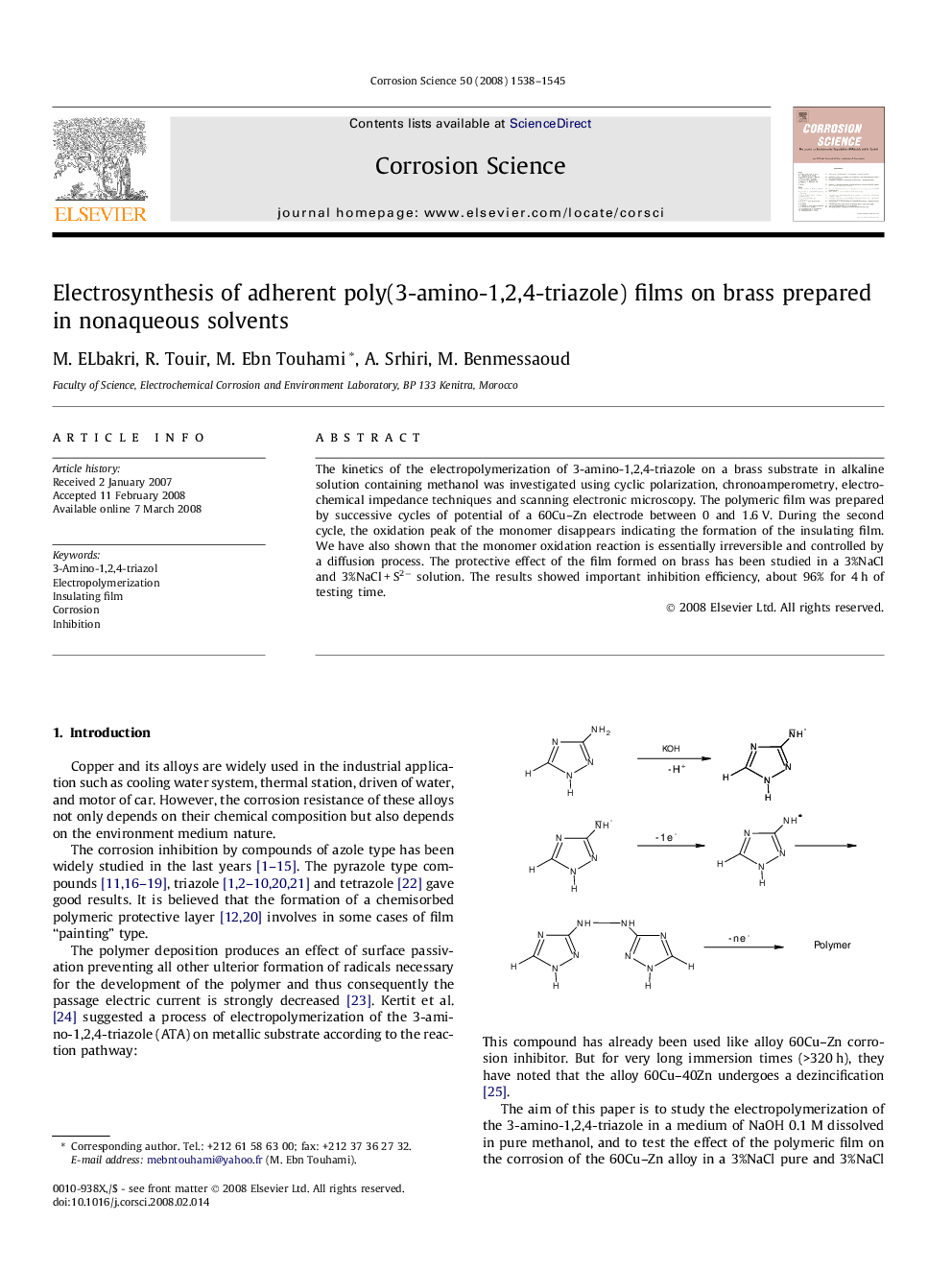
The kinetics of the electropolymerization of 3-amino-1,2,4-triazole on a brass substrate in alkaline solution containing methanol was investigated using cyclic polarization, chronoamperometry, electrochemical impedance techniques and scanning electronic microscopy. The polymeric film was prepared by successive cycles of potential of a 60Cu-Zn electrode between 0 and 1.6 V. During the second cycle, the oxidation peak of the monomer disappears indicating the formation of the insulating film. We have also shown that the monomer oxidation reaction is essentially irreversible and controlled by a diffusion process. The protective effect of the film formed on brass has been studied in a 3%NaCl and 3%NaCl + S2â solution. The results showed important inhibition efficiency, about 96% for 4 h of testing time.
Related Topics
Physical Sciences and Engineering
Materials Science
Ceramics and Composites
Authors
M. ELbakri, R. Touir, M. Ebn Touhami, A. Srhiri, M. Benmessaoud,