| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1520543 | Materials Chemistry and Physics | 2016 | 6 Pages |
•Alkali and alkaline-earth metal atoms form stronger bonds with stanene compared to other group IV monolayers.•Semi-metallic stanene becomes nonmagnetic metal for Li, Na, K, Mg, and Ca atoms adsorption.•Semi-metallic stanene becomes nonmagnetic semiconductor with 94 meV band gap for Be atom adsorption.
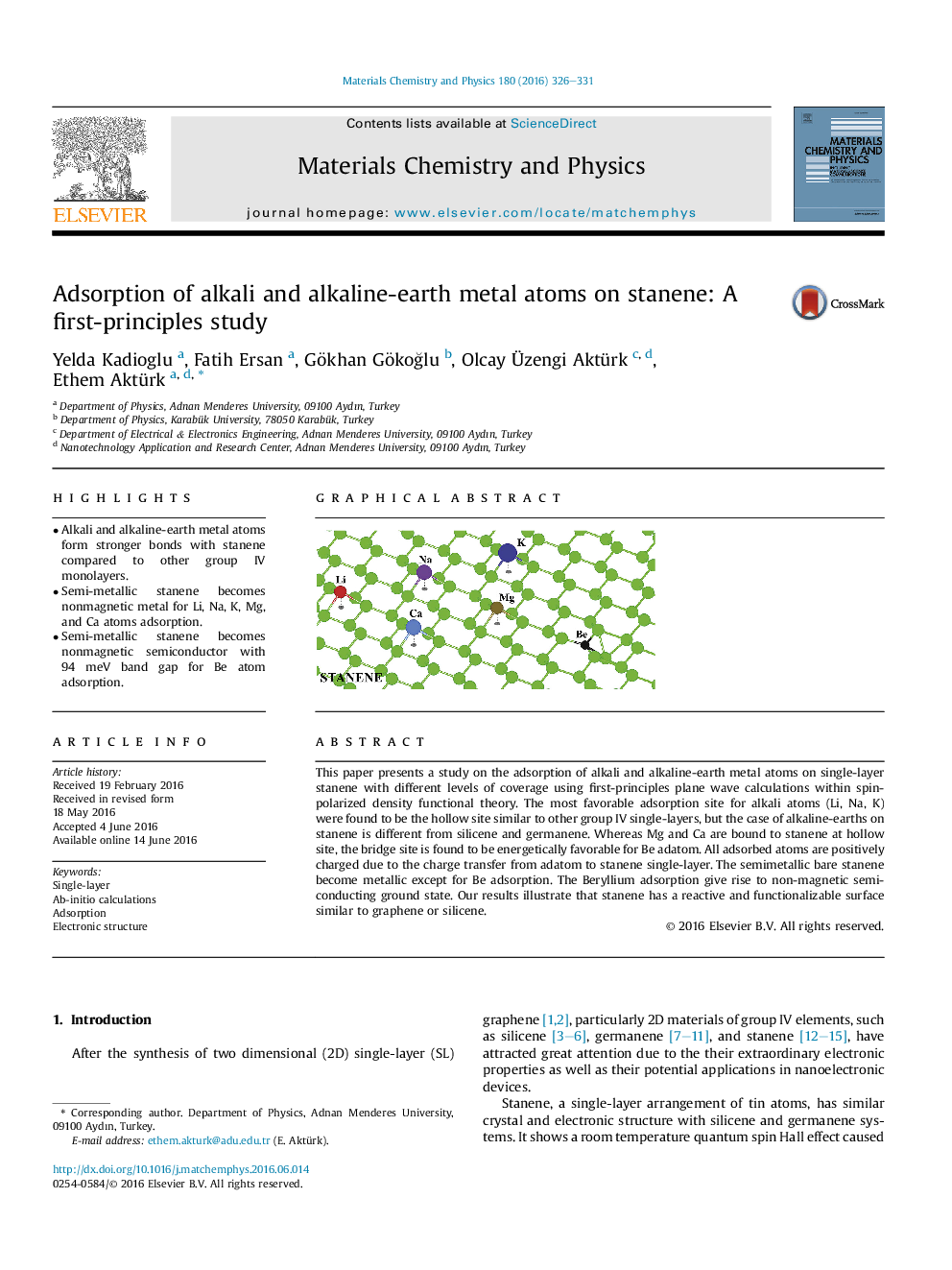
This paper presents a study on the adsorption of alkali and alkaline-earth metal atoms on single-layer stanene with different levels of coverage using first-principles plane wave calculations within spin-polarized density functional theory. The most favorable adsorption site for alkali atoms (Li, Na, K) were found to be the hollow site similar to other group IV single-layers, but the case of alkaline-earths on stanene is different from silicene and germanene. Whereas Mg and Ca are bound to stanene at hollow site, the bridge site is found to be energetically favorable for Be adatom. All adsorbed atoms are positively charged due to the charge transfer from adatom to stanene single-layer. The semimetallic bare stanene become metallic except for Be adsorption. The Beryllium adsorption give rise to non-magnetic semiconducting ground state. Our results illustrate that stanene has a reactive and functionalizable surface similar to graphene or silicene.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide