| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1521842 | Materials Chemistry and Physics | 2014 | 7 Pages |
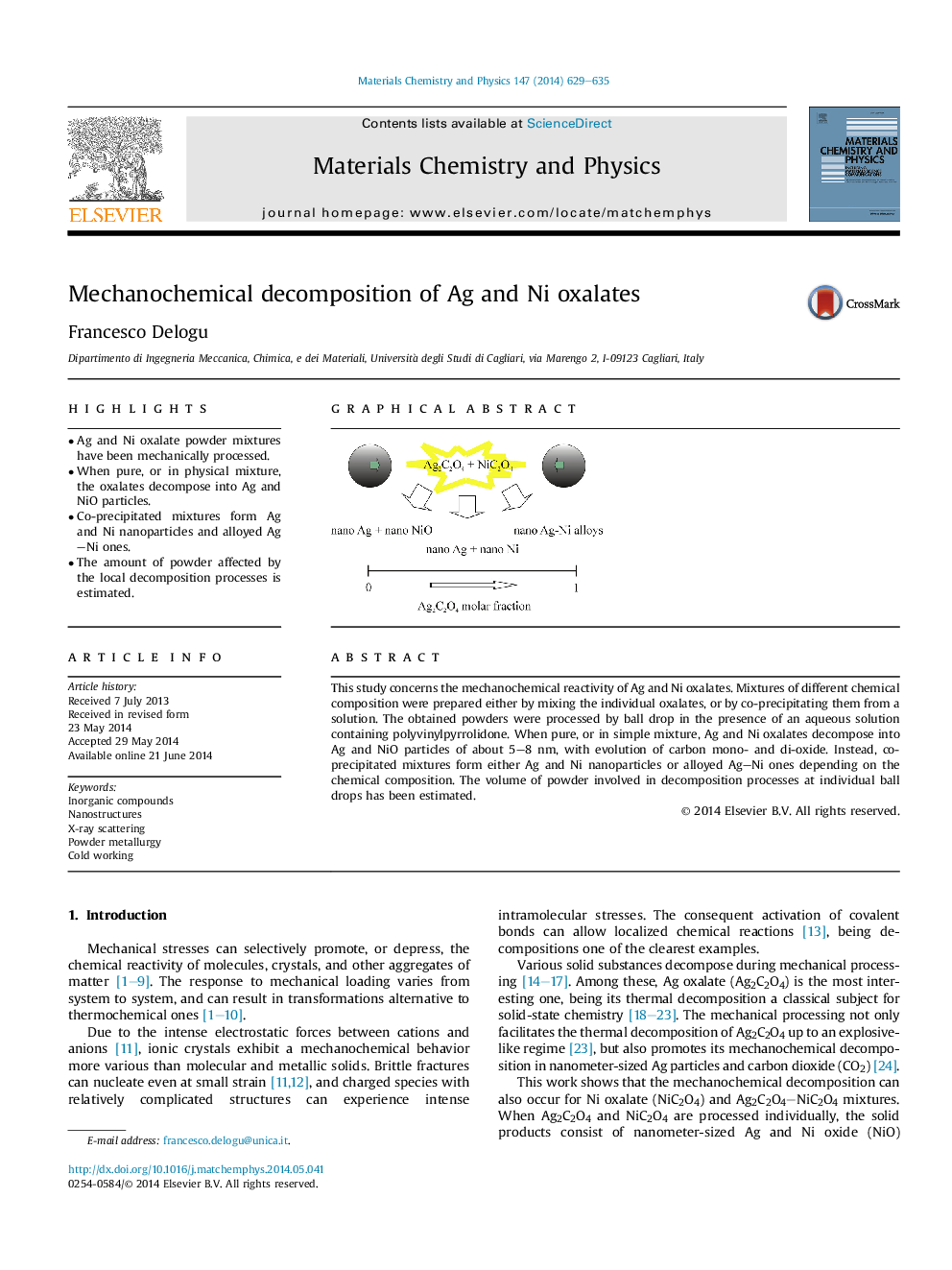
•Ag and Ni oxalate powder mixtures have been mechanically processed.•When pure, or in physical mixture, the oxalates decompose into Ag and NiO particles.•Co-precipitated mixtures form Ag and Ni nanoparticles and alloyed Ag–Ni ones.•The amount of powder affected by the local decomposition processes is estimated.
This study concerns the mechanochemical reactivity of Ag and Ni oxalates. Mixtures of different chemical composition were prepared either by mixing the individual oxalates, or by co-precipitating them from a solution. The obtained powders were processed by ball drop in the presence of an aqueous solution containing polyvinylpyrrolidone. When pure, or in simple mixture, Ag and Ni oxalates decompose into Ag and NiO particles of about 5–8 nm, with evolution of carbon mono- and di-oxide. Instead, co-precipitated mixtures form either Ag and Ni nanoparticles or alloyed Ag–Ni ones depending on the chemical composition. The volume of powder involved in decomposition processes at individual ball drops has been estimated.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide