| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1523854 | Materials Chemistry and Physics | 2012 | 6 Pages |
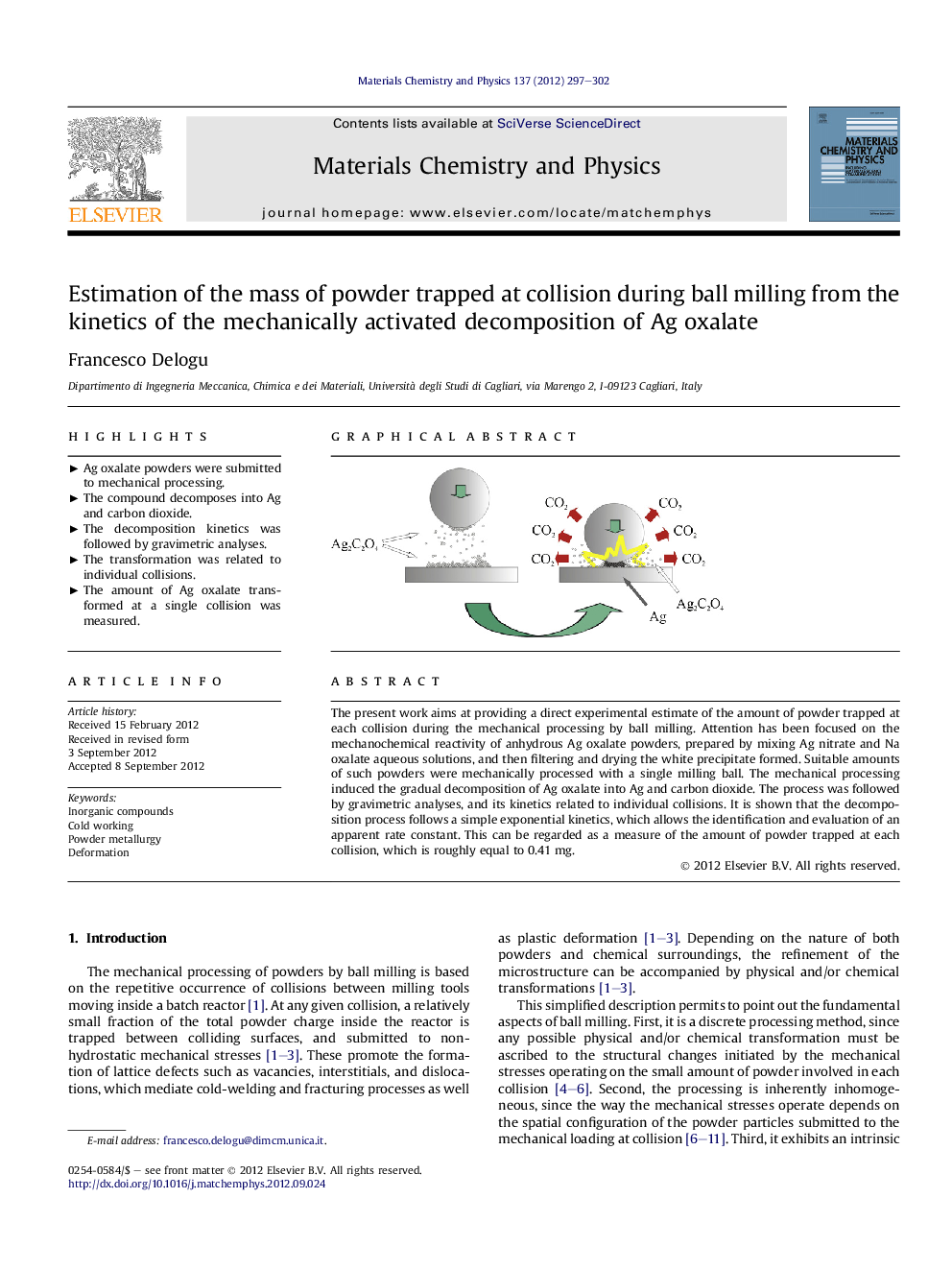
The present work aims at providing a direct experimental estimate of the amount of powder trapped at each collision during the mechanical processing by ball milling. Attention has been focused on the mechanochemical reactivity of anhydrous Ag oxalate powders, prepared by mixing Ag nitrate and Na oxalate aqueous solutions, and then filtering and drying the white precipitate formed. Suitable amounts of such powders were mechanically processed with a single milling ball. The mechanical processing induced the gradual decomposition of Ag oxalate into Ag and carbon dioxide. The process was followed by gravimetric analyses, and its kinetics related to individual collisions. It is shown that the decomposition process follows a simple exponential kinetics, which allows the identification and evaluation of an apparent rate constant. This can be regarded as a measure of the amount of powder trapped at each collision, which is roughly equal to 0.41 mg.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Ag oxalate powders were submitted to mechanical processing. ► The compound decomposes into Ag and carbon dioxide. ► The decomposition kinetics was followed by gravimetric analyses. ► The transformation was related to individual collisions. ► The amount of Ag oxalate transformed at a single collision was measured.