| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 154517 | Chemical Engineering Science | 2016 | 6 Pages |
•Synergetic inhibition of a ionic liquid on hydrate formation when mixed with PVCap.•13C NMR spectra for both structures I and II hydrates with inhibitors.•Time-resolved spectroscopic and macroscopic measurements are also performed.•An ionic liquid shows the synergetic inhibition effect with a commercial inhibitor, PVCap.
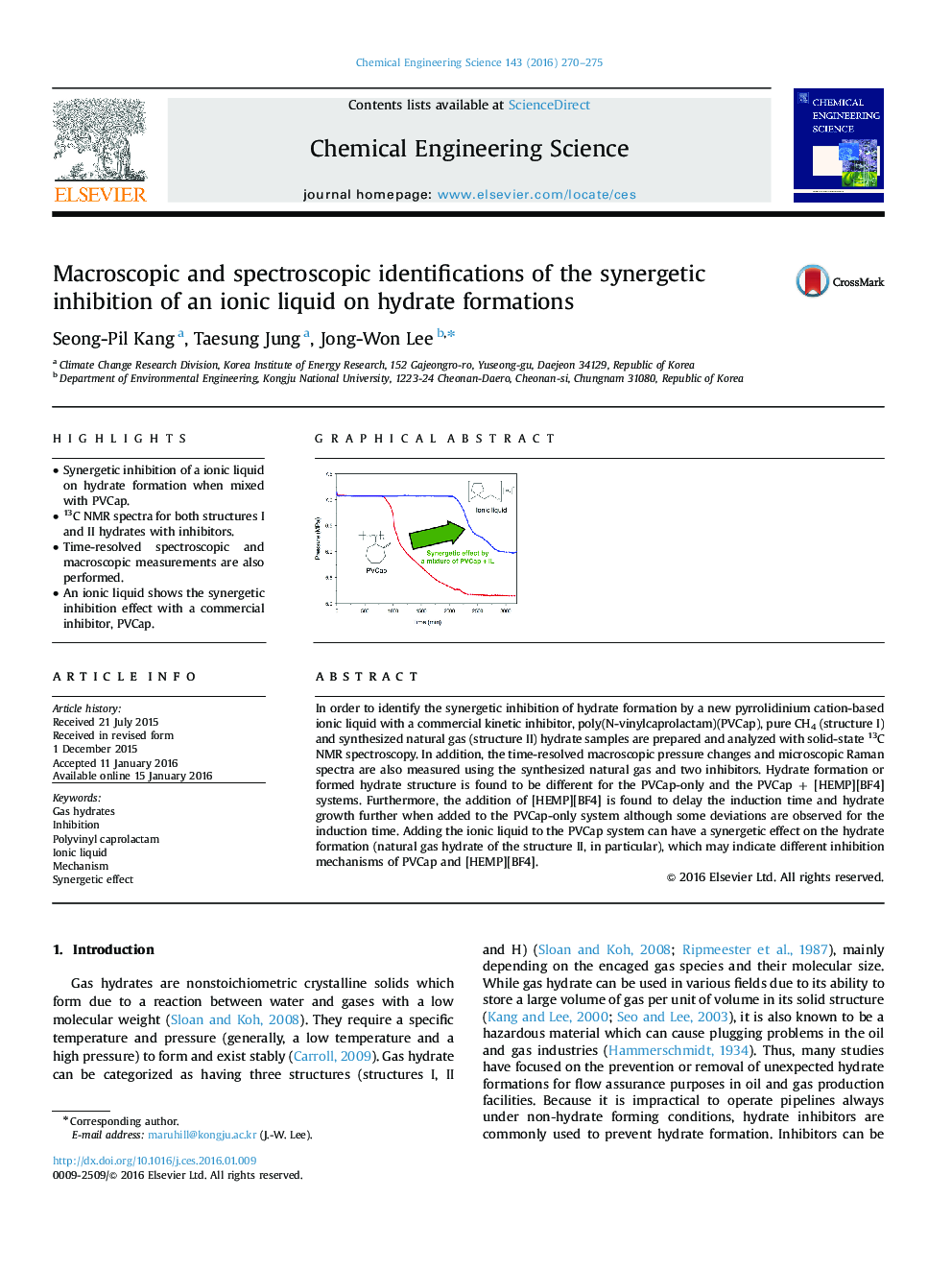
In order to identify the synergetic inhibition of hydrate formation by a new pyrrolidinium cation-based ionic liquid with a commercial kinetic inhibitor, poly(N-vinylcaprolactam)(PVCap), pure CH4 (structure I) and synthesized natural gas (structure II) hydrate samples are prepared and analyzed with solid-state 13C NMR spectroscopy. In addition, the time-resolved macroscopic pressure changes and microscopic Raman spectra are also measured using the synthesized natural gas and two inhibitors. Hydrate formation or formed hydrate structure is found to be different for the PVCap-only and the PVCap + [HEMP][BF4] systems. Furthermore, the addition of [HEMP][BF4] is found to delay the induction time and hydrate growth further when added to the PVCap-only system although some deviations are observed for the induction time. Adding the ionic liquid to the PVCap system can have a synergetic effect on the hydrate formation (natural gas hydrate of the structure II, in particular), which may indicate different inhibition mechanisms of PVCap and [HEMP][BF4].
Graphical abstractFigure optionsDownload full-size imageDownload high-quality image (130 K)Download as PowerPoint slide