| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 155165 | Chemical Engineering Science | 2013 | 9 Pages |
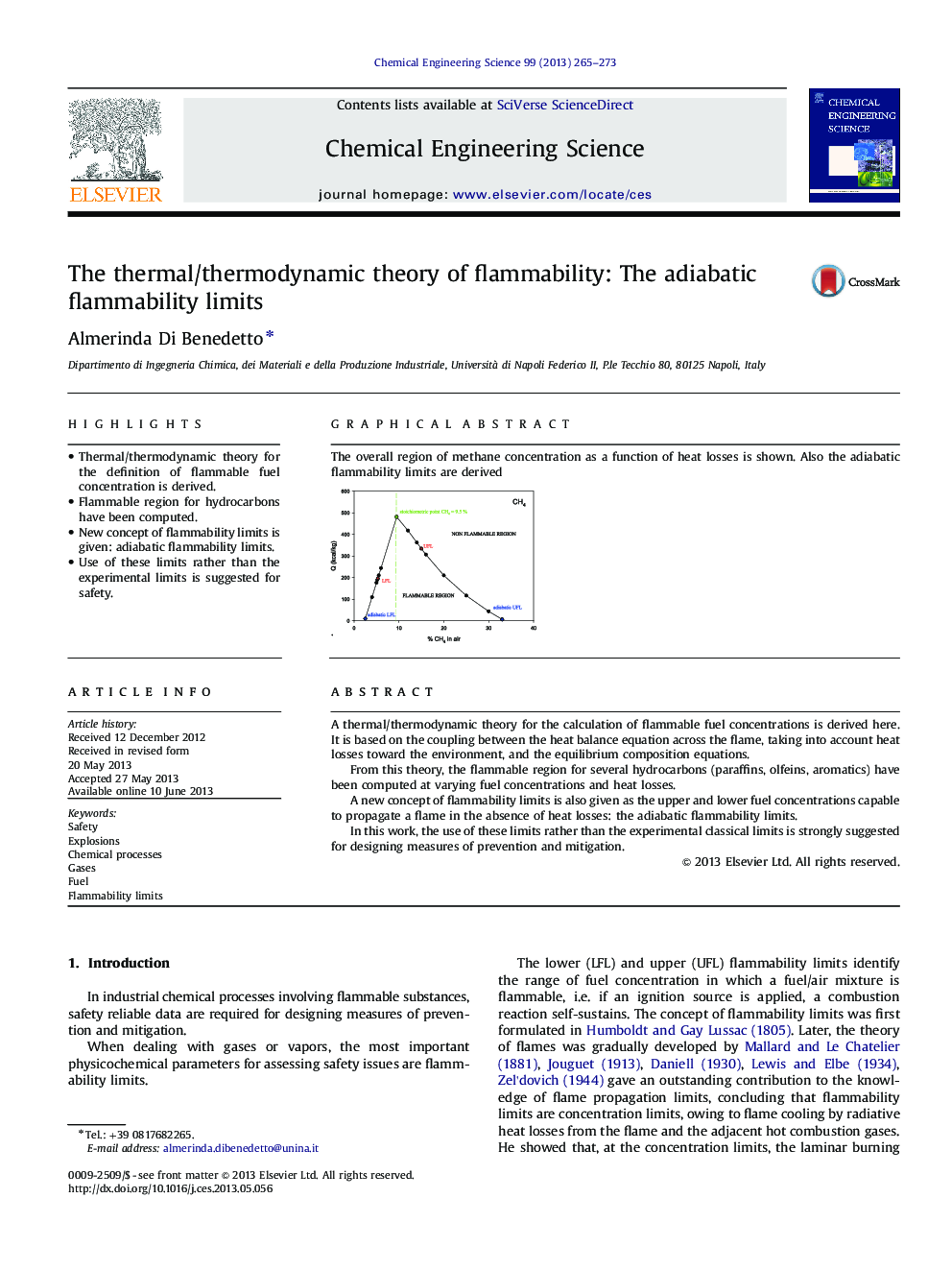
highlights•Thermal/thermodynamic theory for the definition of flammable fuel concentration is derived.•Flammable region for hydrocarbons have been computed.•New concept of flammability limits is given: adiabatic flammability limits.•Use of these limits rather than the experimental limits is suggested for safety.
A thermal/thermodynamic theory for the calculation of flammable fuel concentrations is derived here. It is based on the coupling between the heat balance equation across the flame, taking into account heat losses toward the environment, and the equilibrium composition equations.From this theory, the flammable region for several hydrocarbons (paraffins, olfeins, aromatics) have been computed at varying fuel concentrations and heat losses.A new concept of flammability limits is also given as the upper and lower fuel concentrations capable to propagate a flame in the absence of heat losses: the adiabatic flammability limits.In this work, the use of these limits rather than the experimental classical limits is strongly suggested for designing measures of prevention and mitigation.
Graphical abstractThe overall region of methane concentration as a function of heat losses is shown. Also the adiabatic flammability limits are derived .Figure optionsDownload full-size imageDownload high-quality image (83 K)Download as PowerPoint slide